Bipolar membranes (BPMs) are a specialized type of ion-exchange membrane used in electrochemical processes, particularly for energy conversion, water treatment, and electrolysis applications. The efficiency of these membranes hinges on a combination of material properties, interface characteristics, and operational durability including mechanical strength, ion conductivity, selectivity and permselectivity, and water splitting efficiency. Electrospinning techniques are being used to enhance the performance of these bipolar membranes by modulating several of the membrane properties.

What are Bipolar Membranes?
Bipolar membranes are a unique type of membrane that generates an electrochemical potential across their structure. These membranes consist of two distinct layers, each with different ion-exchange properties, typically an anion-exchange layer (AEL) and a cation-exchange layer (CEL), separated by an interfacial region that allows for the generation of electric potential. At the core of the bipolar membrane’s functionality is the interface between the AEL and CEL. The anion-exchange layer allows the passage of negatively charged ions (anions), while the cation-exchange layer permits the passage of positively charged ions (cations). The interface generates an electric field strong enough to facilitate the splitting of water into hydrogen and hydroxide ions, a process crucial for applications like electrodialysis and electrosynthesis. These membranes have widespread applications including electrodialysis, water desalination, and electrolysis applications.
What Characteristics Are Important in Bipolar Membranes for Efficacy?
For a bipolar membrane to function effectively, several key characteristics must be optimized:
Water Splitting Efficiency
The interfacial region between the anion and cation layers should be designed to efficiently promote the dissociation of water into hydrogen and hydroxide ions. It enables the generation of a pH gradient across the membrane and is critical in electrodialysis and electrolysis systems. This process is at the core of the BPM’s functionality, and any inefficiency here can result in reduced overall performance.
Ion Conductivity
Both the anion-exchange and cation-exchange layers need to have high ionic conductivity to ensure efficient ion-transport. Poor conductivity can result in reduced overall performance and higher energy consumption. The thickness of AEL, CEL and catalytic layers can have an impact on ion conductivity.
Mechanical Strength
Bipolar membranes need to be mechanically robust to handle the pressures and stresses associated with the electrochemical reactions occurring across them. Fragile membranes can result in degradation and can lead to failure.
Selectivity & Permselectivity
The ability of the membrane to selectively transport ions while preventing the diffusion of unwanted ions is crucial. This characteristic ensures that the desired electrochemical reaction occurs efficiently, particularly in processes like electrodialysis. Polymer morphology and hydrophobic/hydrophilic domain tuning to suppress gas solubility and diffusion are key influencers of this characteristic of BPM’s.
Stability & Durability
Given the aggressive environments in which BPMs operate, their stability over extended periods is important. This includes resistance to chemical degradation, fouling, and degradation under high current densities. The composition of the polymers, their resistance to swelling and reinforcement strategies such as the use of porous substrates all play a key role in ensuring stability and durability.
How Can Electrospinning Be Used to Form More Effective Bipolar Membranes?
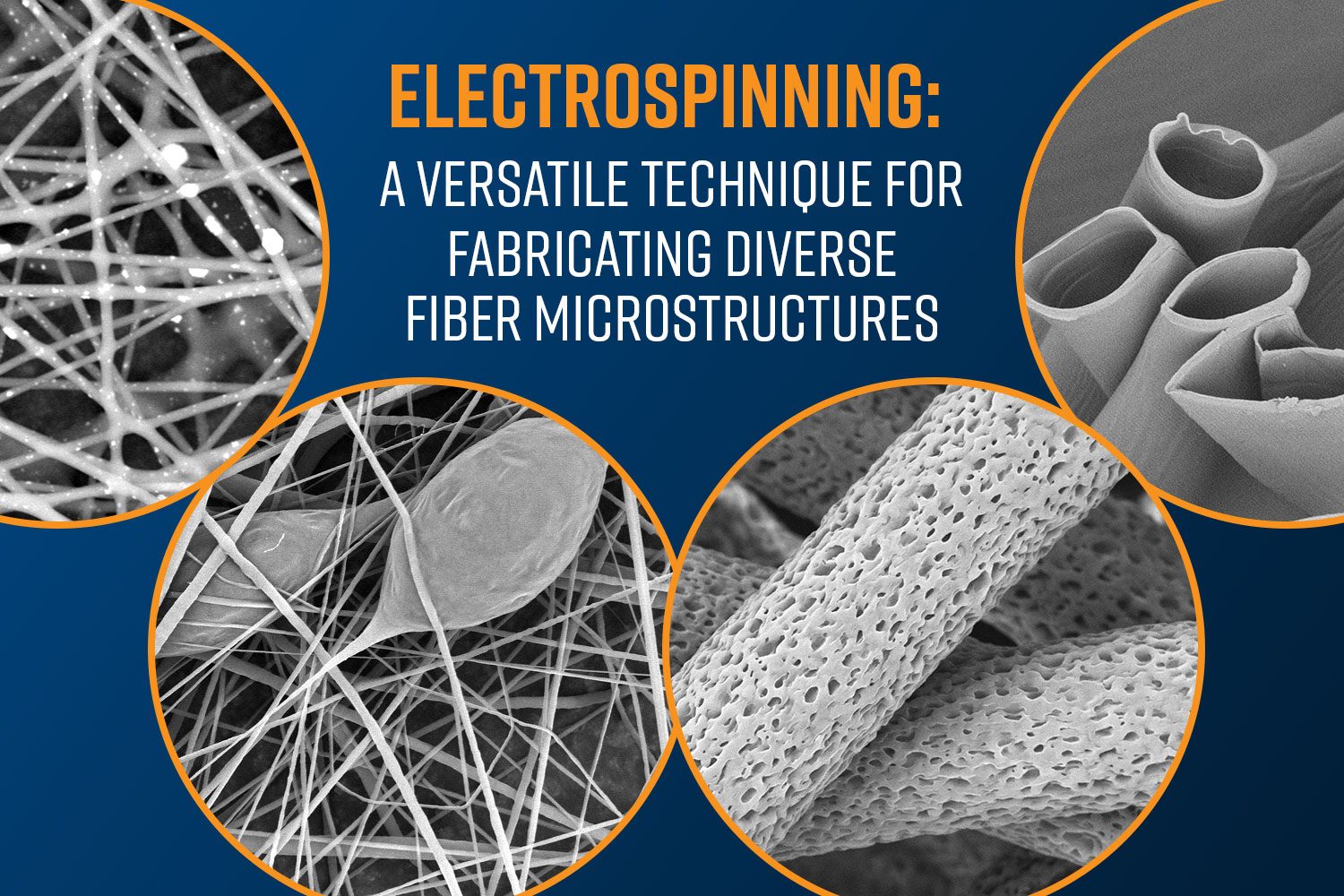
The process of electrospinning and electrospraying provides a promising method for enhancing the structure and performance of bipolar membranes. Electrospinning involves the use of electrostatic forces to fabricate polymeric fibers from a solution, while electrospraying can generate fine droplets or particles that can form thin coatings. These techniques offer unique opportunities to tailor membrane structure and performance by precisely controlling fiber morphology, orientation, and composition. Electrospinning and electrospraying can be used for the fabrication of highly porous, thin, and uniform layers, that can significantly influence key characteristics of BPMs, such as water dissociation efficiency, ion transport, mechanical integrity, and interfacial adhesion.
Electrospinning can be used to fabricate hybrid membranes, where functional additives or nanoparticles are incorporated into the polymeric matrix. This approach can further enhance properties such as ionic conductivity, chemical stability, and resistance to fouling (Figure 1). For example, incorporating nanomaterials such as graphene oxide or titanium oxide can significantly increase the ionic conductivity of the resulting BPMs.
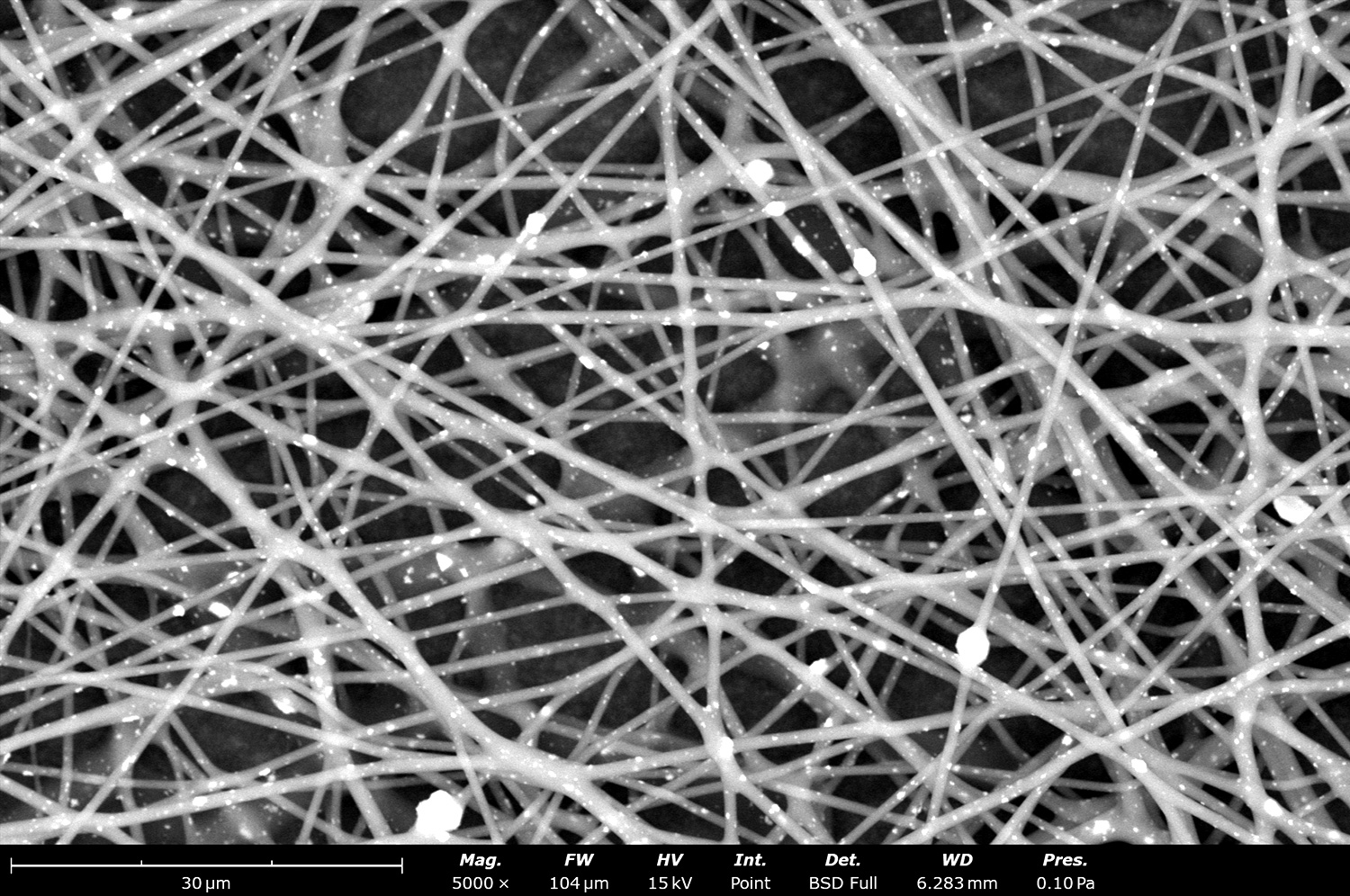

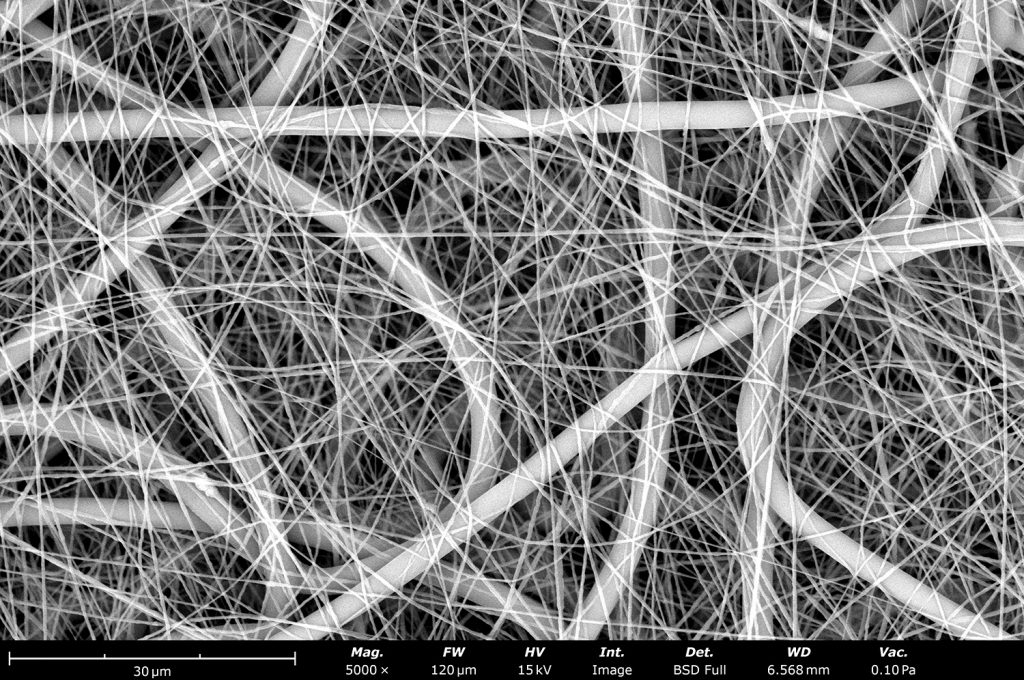
Recent studies have demonstrated the feasibility of using electrospinning and electrospraying to create advanced bipolar membranes. In a study published in ACS Applied Materials & Interfaces,1 electrospun bipolar membranes demonstrated enhanced ion conductivity and stability compared to conventional BPMs. The electrospinning process allowed for the precise control of fiber morphology and material deposition, which in turn improved the membrane’s mechanical properties and ion transport efficiency. Combining this electrospinning with electrospraying allowed seamless incorporation of new catalysts, like nanostructured silica, for an overall novel and highly effective BPM. The researchers used a Fluidnatek LE-50 which enables the production of membranes with uniform fiber structures, essential for achieving high ionic conductivity. The LE-50 is a multi-functional platform that enables users to easily electrospin and/or electrospray simultaneously for a variety of fiber and particle combinations, including effective catalyst incorporation for enhanced materials. Similar and larger samples can also be made with the Fluidnatek LE-100 as shown in Figure 2. The thickness of the electrospun layers, combined with their high porosity, allows for more efficient ion transport across the membrane while maintaining the necessary mechanical strength to withstand electrochemical stress.
Electrospraying, on the other hand, can be used to apply thin coatings of ion-exchange materials onto the surface of a membrane. This technique allows for precise control of layer thickness and composition, leading to improved membrane performance. In a study published in ACS Applied Energy Materials,2 electrospraying was used to deposit ion-exchange materials, enhancing the catalyst layer homogeneity, uniformity, and subsequently the stability and layer integrity of the resulting BPM.
Conclusion
Electrospinning and electrospraying represent powerful techniques for the fabrication of more effective bipolar membranes, enabling the precise control of membrane morphology and properties. The ability to fine-tune factors such as fiber diameter, porosity, and ion-exchange layer composition leads to membranes that offer enhanced conductivity, stability, and performance. As the demand for more efficient electrochemical processes grows, the role of electrospun and electrosprayed bipolar membranes will become increasingly important, particularly in applications related to energy conversion and water treatment. Through continued research and development, these techniques are poised to significantly impact the performance of bipolar membranes, making them more effective, durable, and cost-efficient for a wide range of applications.
References
- Al-Dhubhani. E., Tedesco. M.,de Vos. W.M., & Saakes. M. Combined Electrospinning–Electrospraying for High-Performance Bipolar Membranes with Incorporated MCM-41 as Water Dissociation Catalysts. ACS Applied Materials & Interfaces 2023 15 (39), 45745. https://doi.org/10.1021/acsami.3c06826. ↩︎
- Al-Dhubhani. E., Swart. H., Borneman. Z., et.al. Entanglement-Enhanced Water Dissociation in Bipolar Membranes with 3D Electrospun Junction and Polymeric Catalyst. ACS Applied Energy Materials 2021 4 (4), 3724. https://doi.org/10.1021/acsaem.1c00151. ↩︎