What is Critical Micelle Concentration?
The Critical Micelle Concentration (CMC) is defined as the concentration of surfactants at which micelles spontaneously form in the bulk of a liquid. To understand the significance of the CMC measurement, it is critical to first have a good understanding of surfactants and how their amphiphilic properties affect their behavior in liquids.
What are surfactants?
Surface-active agents, often abbreviated to surfactants, are molecules that are active at surfaces or interfaces. Surfactants are particularly noteworthy and widely used in a variety of consumer products due to their ability to reduce surface tension at interfaces, facilitating the formation of stable colloidal systems. This applies to a variety of surfaces and interfaces, including solid-liquid interfaces, air-liquid surfaces, or liquid-liquid interfaces. A defining property of a surfactant is that in solution, the surfactant concentration is higher at the liquid’s surface than in the bulk. This tendency is explained by the fact that surfactants are amphiphilic molecules, which means they are comprised of both a hydrophobic (water-repelling) and hydrophilic (water-attracting) component, as shown in Figure 1A. The word amphiphile is derived from the Greek prefix amphi, and the root philos, which can be interpreted as “double,” and “affinity for,” respectively, describing their behavior when in aqueous environments.
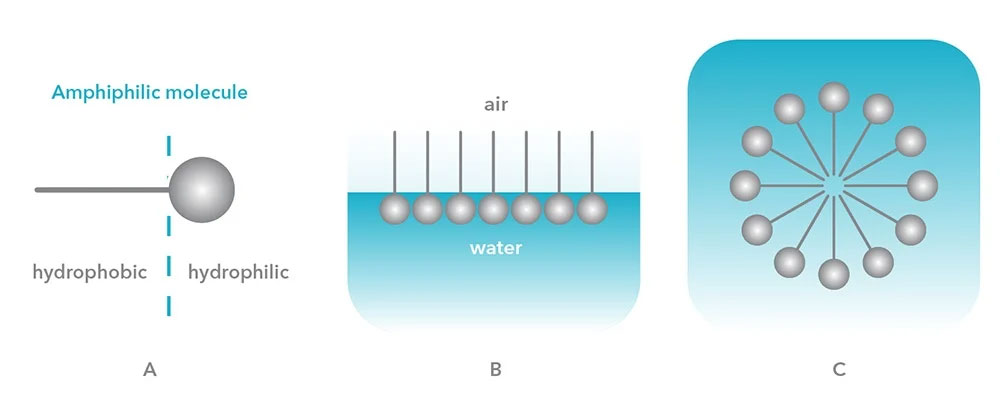
In water (a polar liquid) the polar “head” of the molecule undergoes dipole or ion-dipole interactions with the water molecules, while the hydrocarbon chains that comprise the “tail” of the molecule are repelled by, or only weakly interact with the water. This explains the surfactant molecules’ strong tendency to align themselves at the liquid-air or liquid-solid interface with hydrophobic tails oriented away from the water, which is depicted in Figure 1B. As more molecules align at the surface, the cohesive energy between the water molecules is disrupted, reducing the surface tension between the two phases.
As the concentration of surfactant molecules aligned at the surface continues to increase, the water molecules and hydrophobic “tails” repel each other to such an extent that the cooperative action of dispersion and hydrogen bonds will push the hydrocarbon chains towards each other, leading to self-assembly of aggregates called micelles. This molecular orientation is illustrated in Figure 1C. Depending on the concentration of surfactant in the liquid, different proportions of surfactant molecules will either align at the surface, or form micelles in the bulk. The point at which the surface is crowded with surfactant and micelles begin to form is called the Critical Micelle Concentration (CMC). CMC can be determined using surface tension measurements.
Surface tension isotherms and CMC Measurement
In general, as the surfactant concentration in the bulk phase is increased at constant temperature and pressure, the surface concentration of surfactant (Γ) that is adsorbed increases, resulting in lower surface or interfacial tensions. The relationship between bulk concentration and surface tension at a constant temperature is called an isotherm, and an equation describing this relationship can be derived from the Gibbs adsorption isotherm. One commonly used and relatively simple example is the Szyskowski equation [1],
γ = γ0 – nȒTΓ∞ ln(1+KC)
Equation 1
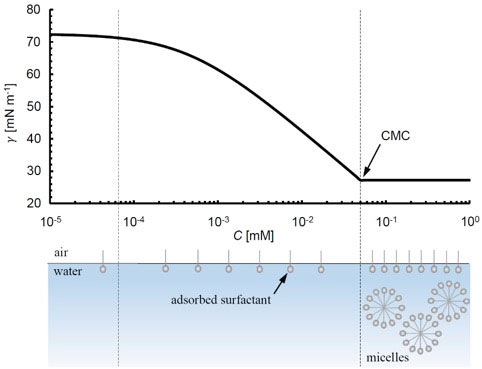
where γ0 is the surface tension without surfactant, Ȓ is the universal gas constant, T is temperature, Γ∞ is the maximum possible surface concentration of surfactant, K is the surface-bulk equilibrium constant, and n is a coefficient that is 1 for non-ionic surfactants such as tetraethylene glycol monododecyl ether (C12E4) [2]. In Figure 2, a plot of eq. 1 for a C12E4 – air surface is shown using K and Γ∞ values from [2].
As the bulk concentration continues to increase, a point is reached where the surface concentration no longer increases. Instead, surfactants in the bulk begin aggregating into micelles. This point is called the critical micelle concentration or CMC and is driven by thermodynamics. The bottom of Figure 2 demonstrates this transition schematically. After the CMC, surface tension tends to not vary significantly with bulk concentration.
References
[1] A.J. Prosser, E.I. Franses. Adsorption and surface tension of ionic surfactants at the air-water interface: review and evaluation of equilibrium models. Colloids Surf. A 178 (2001) p. 1-40.
[2] C.-T. Hsu, M.-J. Shao, S.-Y. Lin. Adsorption kinetics of C12E4 at the air-water interface: adsorption onto a fresh interface. Langmuir 16 (2000) p. 3187-3194.