In medical device R&D, the ability to work with small quantities of high-value or experimental materials is essential. Micro compounding has emerged as a important tool in the development and manufacturing of medical devices as it enables precise formulation and testing of polymer-based materials on a small scale, providing manufacturers with the flexibility to optimize performance, biocompatibility, and regulatory compliance before moving to full-scale production. Micro compounding allows for rapid iteration of formulations with minimal material and waste.

By offering controlled mixing, accurate additive incorporation, and uniform material properties, micro compounding supports the development of devices ranging from drug-eluting stents and implantable devices to tissue scaffolds and specialized coatings. It accelerates the innovation process, reduces costs associated with trial-and-error experimentation, and ensures that early-stage prototypes meet both functional and safety requirements.
| Aspect | Micro Compounding | Conventional Compounding |
| Material Volume | 2 – 50 mL | Several liters |
| Waste | Minimal | High |
| Speed | Fast iteration | Slower, costly adjustments |
| Precision | High control over shear and temperature | Limited, scale-dependent |
| Application | Early-stage R&D, prototyping | Full-scale production |
Key Roles of Micro Compounding in Medical Device Manufacturing
Material Development & Testing:
Medical devices often rely on specialized polymers, elastomers, or composites with unique mechanical, chemical, or biological properties. Micro compounders allow manufacturers to test new polymer blends with small quantities (2 – 50 mL) of material and quickly evaluate multiple formulations to identify optimal material properties. Micro compounding can also enable the assessment of compatibility and functionality after incorporation of additives, drugs, or bioactive agents.
Cost & Resource Efficiency:
Many high-performance polymers and bioactive additives are expensive or available in limited quantities. Micro compounding minimizes waste by requiring only small sample amounts and enables early-stage development without committing to large-scale production. It also reduces cost associated with trial-and-error testing of new formulations.
Controlled Mixing & Homogeneity:
Micro compounders are designed to provide precise shear, temperature control, and residence time, which ensures uniform mixing. Further, set and measured values can be recorded to compare compounding processing conditions to the properties of the extruded material. This is important for optimizing and maintaining consistent mechanical and chemical properties in implants or device components. It is also essential for drug-eluting medical devices where even distribution of active agents affects efficacy.
Rapid Prototyping & Customization:
In medical device R&D, rapid iteration is crucial. Micro compounding facilitates and accelerates the development of device-specific materials in small batches ideal for testing mechanical performance, biocompatibility, and sterilization stability and early evaluation of regulatory requirements such as ISO 10993.
Support for Regulatory & QA Studies:
Micro-compounded batches can be used to generate data for characterization of thermal, mechanical, and chemical properties of the compounded material. Optimized micro compounders offer rapid injection molding capabilities to quickly produce mechanical testing specimens. It can also be used to generate materials required for in- vitro testing of biocompatibility or bioactivity and stability and shelf-life assessments.
Micro Compounding Applications in Medical Device Manufacturing
A few example applications of Micro Compounding in medical device development include the development of the following:
Drug-Eluting Stents:
Drug-eluting stents are advanced cardiovascular implants designed to keep arteries open while delivering therapeutic agents directly to the vessel wall. These stents consist of a metal scaffold coated with a polymer matrix that controls the release of drugs thereby reducing the risk of restenosis after angioplasty. The performance of a drug-eluting stent depends heavily on the uniformity, stability, and release profile of the polymer–drug coating, making precise material formulation a critical part of development.1
Micro compounding can precisely blend polymers with drugs at small scales to optimize release kinetics, ensure homogeneous distribution of the drug within the polymer matrix and test multiple formulations rapidly to identify the most effective combination.2
Catheters and Tubing:
Catheters and medical tubing are essential components across a wide range of diagnostic and therapeutic procedures, requiring materials that balance flexibility, durability, and biocompatibility. These polymer-based structures must withstand mechanical stress, maintain smooth surfaces for safe insertion, and remain stable under repeated stress and sterilization cycles. Additives such as plasticizers, lubricants, or antimicrobial agents are often incorporated to enhance performance and reduce infection risk. Because material consistency directly affects patient safety and device reliability, careful formulation and testing of catheter and tubing polymers are central to medical device development.3
Micro Compounding can be used to produce small batches of polymer compounds by adding plasticizers, lubricants, or antimicrobial agents in controlled amounts to evaluate material properties such as flexibility, surface smoothness, and resistance to degradation without wasting large volumes of polymer. Many micro compounding systems can directly extrude micro tubes to further speed up R&D and test process parameters for upscaling.
Implantable Devices:
Implantable devices rely on carefully engineered polymers and composites that provide long-term mechanical stability, biocompatibility, and resistance to degradation inside the body. These materials must perform reliably under physiological loads while minimizing adverse tissue responses, making precise control over formulation and additive incorporation essential. Reinforcing agents, bioactive fillers, or specialized surface modifiers are often blended into the base polymer to enhance strength, promote tissue integration, or reduce wear. Because the safety and effectiveness of an implant depend directly on its material properties, rigorous small-scale development and optimization remain a foundational step in creating high-performance implantable medical technologies.4
Micro compounding can be used to optimize formulation by combining high-performance polymers with reinforcing agents like fibers or nanoparticles in small quantities and test thermal and mechanical stability under simulated body conditions (Figure 1).

Tissue Engineering Scaffolds:
Tissue engineering scaffolds are designed to provide a temporary, biodegradable framework that supports cell attachment, proliferation, and tissue regeneration. These scaffolds rely on polymers that can be precisely tailored for porosity, mechanical strength, and degradation rate to match the needs of specific tissues. Bioactive molecules, growth factors, or nanoparticles are often incorporated to enhance biological signaling and improve regenerative outcomes. Because scaffold performance depends on both material composition and structural integrity, controlled formulation and small-scale prototyping are essential steps in developing effective, clinically relevant tissue engineering solutions (see Figure 2).5
Micro Compounding can blend biodegradable polymers with growth factors or bioactive nanoparticles to ensure even distribution of components for uniform scaffold properties and enable rapid prototyping of multiple formulations for in vitro and in vivo testing. Micro compounders can support the production of scaffolds that are fabricated by additive manufacturing methods by developing gauged filaments optimized for 3D printing applications. This can be valuable while in formulation or even small-scale production.
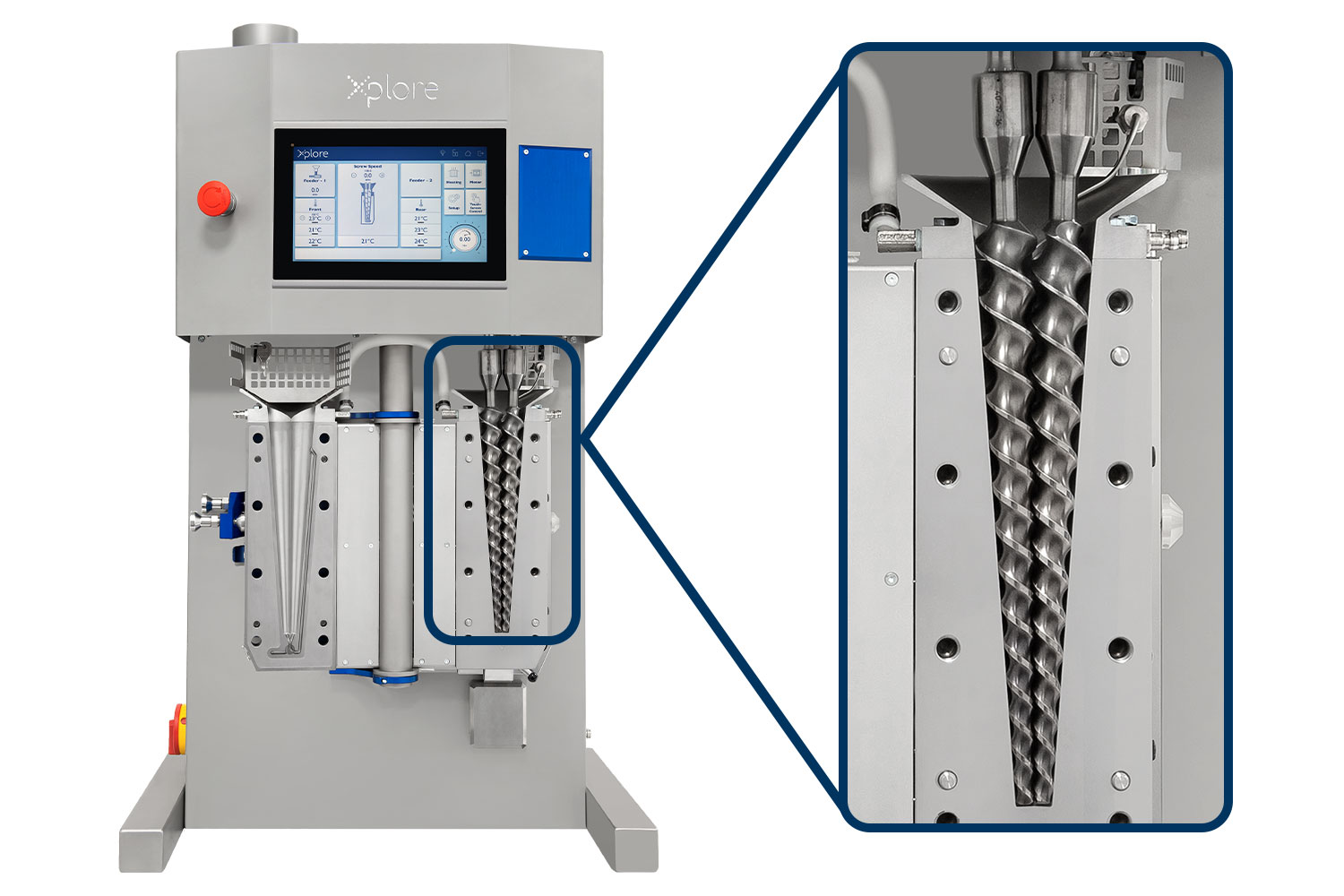
Xplore Micro Compounders:
Xplore micro-compounders are lab-scale systems designed to replicate industrial polymer processing with exceptional precision while using only 2 – 40 mL of material. Their small batch capability is ideal for high-value, experimental, or limited-quantity polymers, enabling efficient formulation work with faster temperature control, minimal waste, and easy cleaning. The fully intermeshing twin-screw design delivers strong shear, continuous mixing, and uniform dispersion of additives or nanoparticles. The system’s tunable residence time that is achieved through a recirculation channel and valve-controlled flow, allows researchers to precisely control shear and thermal exposure. Xplore micro-compounders provide a flexible, efficient platform for a wide range of polymer R&D applications.
Summary
Micro compounding is an essential tool in medical device manufacturing, bridging the gap between material research and full-scale production. It allows precise formulation, efficient use of costly or rare materials, rapid prototyping, and early-stage testing to ensure performance, safety, and regulatory compliance. It allows precise control over polymer formulations and additive incorporation, which is critical in medical devices where material performance directly impacts safety and efficacy. From drug-eluting stents to tissue scaffolds, this technique enables rapid iteration, small-batch testing, and material optimization, accelerating the development of innovative and compliant medical products.
References
- Sindeeva, O. A.; Prikhozhdenko, E. S.; Schurov, I.; Sedykh, N.; Goriainov, S.; Karamyan, A.; Mordovina, E. A.; Inozemtseva, O. A.; Kudryavtseva, V.; Shchesnyak, L. E.; Abramovich, R. A.; Mikhajlov, S.; Sukhorukov, G. B. Patterned Drug-Eluting Coatings for tracheal stents based on PLA, PLGA, and PCL for the granulation formation reduction: in vivo studies. Pharmaceutics 2021, 13 (9), 1437. https://doi.org/10.3390/pharmaceutics13091437. ↩︎
- Ghaffari, A.; Matter, B. A.; Hartman, R. R.; Bourne, D. W. A.; Wang, Y.; Choi, S.; Kompella, U. B. Hot-Melt Extrusion-Based Dexamethasone–PLGA implants: physicochemical, physicomechanical, and surface morphological properties and in vitro release corrected for drug degradation. Pharmaceutics 2024, 16 (7), 895. https://doi.org/10.3390/pharmaceutics16070895. ↩︎
- Koyama, S.; Haniu, H.; Osaka, K.; Koyama, H.; Kuroiwa, N.; Endo, M.; Kim, Y. A.; Hayashi, T. Medical application of Carbon‐Nanotube‐Filled nanocomposites: the microcatheter. Small 2006, 2 (12), 1406–1411. https://doi.org/10.1002/smll.200500416. ↩︎
- Tabrizian, P.; Sun, H.; Jargalsaikhan, U.; Sui, T.; Davis, S.; Su, B. Biomimetic Nacre-Like Hydroxyapatite/Polymer composites for bone implants. Journal of Functional Biomaterials 2023, 14 (8), 393. https://doi.org/10.3390/jfb14080393. ↩︎
- Sharma, S.; Sudhakara, P.; Singh, J.; Ilyas, R. A.; Asyraf, M. R. M.; Razman, M. R. Critical review of biodegradable and bioactive polymer composites for bone tissue engineering and drug delivery applications. Polymers 2021, 13 (16), 2623. https://doi.org/10.3390/polym13162623. ↩︎