Per- and PolyFluoroAlkyl Substances (PFAS) have been widely used in a range of applications. The concern with PFAS arises from their persistence in the environment and potential adverse health effects. To address the concerns associated with PFAS, initiatives are being implemented to develop remediation strategies. Adsorption, where PFAS attaches to the surface of a material through weak Van der Waals forces, is one of the methods being investigated to remove PFAS from water.
Quartz Crystal Microbalance with Dissipation monitoring (QCM-D) is a versatile analytical technique that can be used to study the adsorption behavior of PFAS on solid surfaces. By coating a quartz crystal with the material of interest and monitoring changes in frequency and dissipation, researchers can measure the adsorption mass and kinetics on the surface. This provides insights into the interactions between PFAS and different surfaces, such as coatings and membranes, with potential applications in PFAS remediation.

Per- and PolyFluoroAlkyl substances (PFAS)
Per- and polyfluoroalkyl substances (PFAS) are a diverse group of synthetic chemicals used in industrial and consumer products since the 1940s. PFAS have been widely used for their heat, water, and oil-resistant properties and are ingredients in many everyday products. For example, PFAS are used to make non-stick cookware and stain-resistant clothing and carpets. They are characterized by their chemical structure of linked carbon and fluorine atoms.
While the chemical properties of PFAS make them useful in a variety of industries, they are also causing growing concern for the environment and public health. Due to the strength of their carbon-fluorine bonds, PFAS do not degrade easily in the environment. Their environmental persistence combined with their extensive use in the past decades has resulted in PFAS being found in drinking water, soil, and blood of people and animals around the world. Current scientific research, while still ongoing, suggests that exposure to certain PFAS, even at very low levels, may cause adverse health outcomes in humans and animals. The health risks associated with PFAS exposure can vary depending on various factors including the specific PFAS compound, the level and duration of exposure, and individual susceptibility. In response to new scientific research, the EPA issued a final regulatory determination to regulate two PFAS, perfluorooctanoic acid (PFOA) and perfluorooctane sulfonic acid (PFOS), as contaminants under the Safe Drinking Water Act in March 2021. In March 2023, the EPA proposed a National Primary Drinking Water Regulation to establish legally enforceable levels of 4.0 parts per trillion (ng/L) for PFOA and PFOS.
Quartz Crystal Microbalance with Dissipation Monitoring (QCM-D)
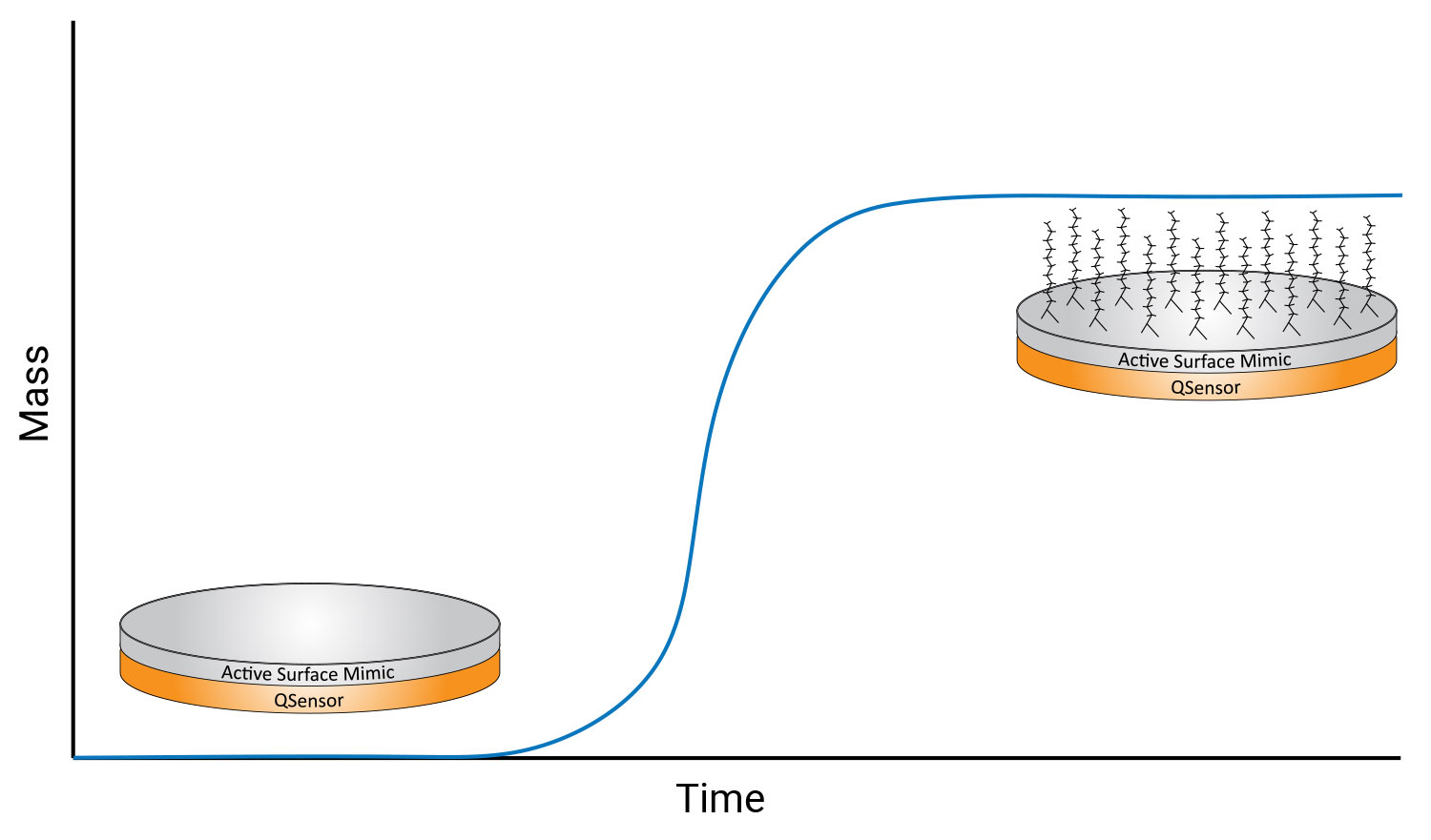
QCM-D is a surface-sensitive, label-free technique that measures mass changes at the surface with a sensitivity of <0.3 ng/cm2. The time-resolved information can be used to analyze molecule-surface interactions such as adsorption, desorption, swelling, crosslinking, and etching.
QCM-D monitors the resonance frequency of a quartz sensor as a function of time. The resonance frequency of the sensor depends on its mass, so changes in frequency will reveal changes in the mass coupled to the sensor surface. Energy loss of the system, or dissipation, is also monitored by QCM-D. Changes in dissipation can be used to quantify the viscoelastic properties of soft layers attached to the sensor. Frequency and dissipation changes can be used to analyze molecular interactions with the sensor surface.
A typical QCM-D sensor has a pair of gold metal electrodes deposited on the top and bottom of a quartz disc to oscillate the quartz. While molecular interactions can be studied directly with the gold surface, the surface can also be coated with other materials to mimic real surfaces of interest. For example, the sensor can be coated with a polymer to study the binding kinetics of PFAS.
Engineered Sorbents for PFAS Removal
In a recent research study, Lee et. al. explored the ability of surface-engineered iron oxide nanocrystals (IONCs) to remove PFAS from water using QCM-D to study the real-time sorption dynamics of PFAS with IONC surfaces.1 In this study, different IONC layers were formed on silica-coated QCM-D sensors and exposed to different concentrations of PFOA and PFOS. Sorption isotherms were determined from the QCM-D data and used to compare the sorption capacity of the different IONCs. The binding efficiency of PFOA in the presence of PFOS and vice versa was also investigated. The QCM-D data revealed that the sorption of PFOA was significantly hindered by the presence of PFOS. However, PFOS sorption was not as hindered by the presence of PFOA, but still had reduced sorption density. These results suggest that steric hindrance, along with other complicating factors affect sorption capacities in multi-sorbate systems.
Kinetics of Adsorption of PFAS on Hydrophobic Surfaces
Adsorption plays an important role in determining the fate of PFAS in the environment as well as developing PFAS removal processes for water treatment. However, predicting the sorption of PFAS based on surface and solution conditions remains difficult due to the complexity of PFAS-surface interactions. Mohona et. al. examined the absorption behavior of PFAS on a hydrophobic surface using QCM-D to elucidate the hydrophobic interaction between PFAS and the surface.2 Sensors coated with a hydrophobic CH3-terminated self-assembled monolayer (SAM) were exposed to different concentrations of perfluorononanoic acid (PFNA) and PFOS. The absorbed mass determined from the QCM-D data was used for kinetic modeling.
The absorption kinetics of PFNA and PFOS on the CH3-terminated SAM revealed two distinct kinetic regions, suggesting that there were different dominant interaction mechanisms as the absorption progressed over time. PFNA and PFOS behaved similarly during the initial absorption stage, where the PFNA/PFOS molecules likely directly interacted with the SAM surface. During the second stage, the kinetic model suggested that the absorption mechanisms started to diverge between PFNA and PFOS. The QCM-D experiments showed that both PFNA and PFOS had multistage absorption kinetics on CH3-terminated SAM and that PFNA had a higher absorption capacity than PFOS.
Conclusion
Overall, QCM-D is a valuable tool for investigating the adsorption behavior, surface modification, and interactions of PFAS on various surfaces. It helps in understanding the fundamental aspects of PFAS interactions with different materials and provides insights into potential strategies for PFAS mitigation and remediation.
References
[1] Lee, J., Kim, C., Liu, C., Wong, M.S., Cápiro, N.L., Pennell, K.D., Fortner, J.D. Ultra-high capacity, multifunctional nanoscale sorbents for PFOA and PFOS treatment. npj Clean Water 2023 6 (62) https://doi.org/10.1038/s41545-023-00263-9
[2] Mohona, T.M., Ye, Z., Dai, N., Nalam, P.C. Adsorption behavior of long-chain perfluoroalkyl substances on hydrophobic surface: A combined molecular characterization and simulation study. Water Research 2023 239 (120074) https://doi.org/10.1016/j.watres.2023.120074