Pharmaceutical & Drug Research
Solutions for Pharmaceuticals
In addition to the value contributed to human health and quality of life, the global market for pharmaceuticals was valued at close to $1.5 trillion in 2022. Empowering research and development in this field with precise and powerful instrumentation is essential for the discovery of novel drugs and optimization of current ones. From initial compounding to purification, storage, and delivery, there are many steps of the production process that could benefit from focused experimentation.
Nanoscience Instruments provides advanced solutions for every step of the pharmaceutical development process, including:
Small volume compounding
Biosensor development
Nanofiber / nanoparticle drug encapsulation
Microsphere cross sectioning
Morphological analysis and impurity detection
Automated SEM workflows
Wettability and surface tension measurements
Interfacial interaction analysis
Solutions for Pharmaceuticals
Our Solutions

SEMPREP SMART
SEMPREP SMART is an automated broad ion milling system featuring two ion sources, low-energy and high-energy. This enables the SEMPREP SMART to have the widest range and highest precision of ion mills on the market. For certain samples, this also eliminates the need for cooling, since they can be milled more gently. Regardless of sample type, the SEMPREP SMART is a great choice for high-quality imaging preparation.

Phenom SEM
The Phenom line of desktop SEMs hosts the most powerful and reliable electron sources on the market, enabling higher resolutions and magnifications while also reducing maintenance costs. Operators can be trained in a matter of hours, if not minutes, and time-to-image can be as short as 30 seconds. Multiple Phenom models with varying capabilities and sample stages are available to match your analysis needs.

QSense
Founded by the original developers of the QCM-D technique, QSense is uniquely equipped to provide top-tier instrumentation to their users. Their technology is more sensitive and easier to use than any QCM instrument on the market, lending value to even the most sensitive and complex experiments. Data analysis can be completed with the accompanying QSoft software.

Attension
The Attension Theta and Attension Sigma are lines of optical and force tensiometers, respectively. In addition to class-leading hardware and all-inclusive software, the Attension instruments are fully modular, with various plug-and-play accessories available for complex experimentation. The robust instrument design and option to use chemically resistant pipette tips enables use inside of a glovebox and measurements with harsh chemicals.
Fluidnatek
From benchtop systems to fully automated, industrial-size manufacturing, the Fluidnatek and Spinbox electrospinning systems offer flexibility and scalability for every application. Their focus on environmental control leads to consistency, precision, and efficiency throughout nanofiber production. This is particularly valuable in research applications, where reproducibility is critical for verifying observations and trends.
Solutions for Pharmaceuticals
Drug Discovery
Small Volume Compounding
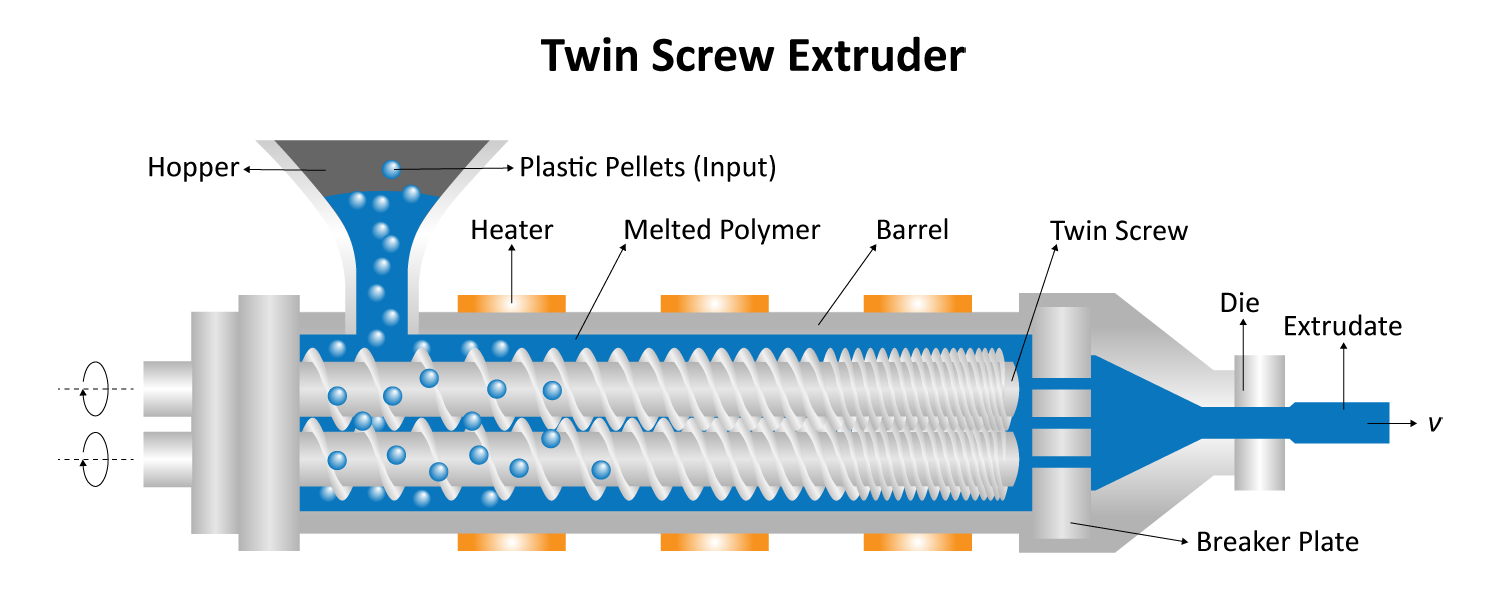
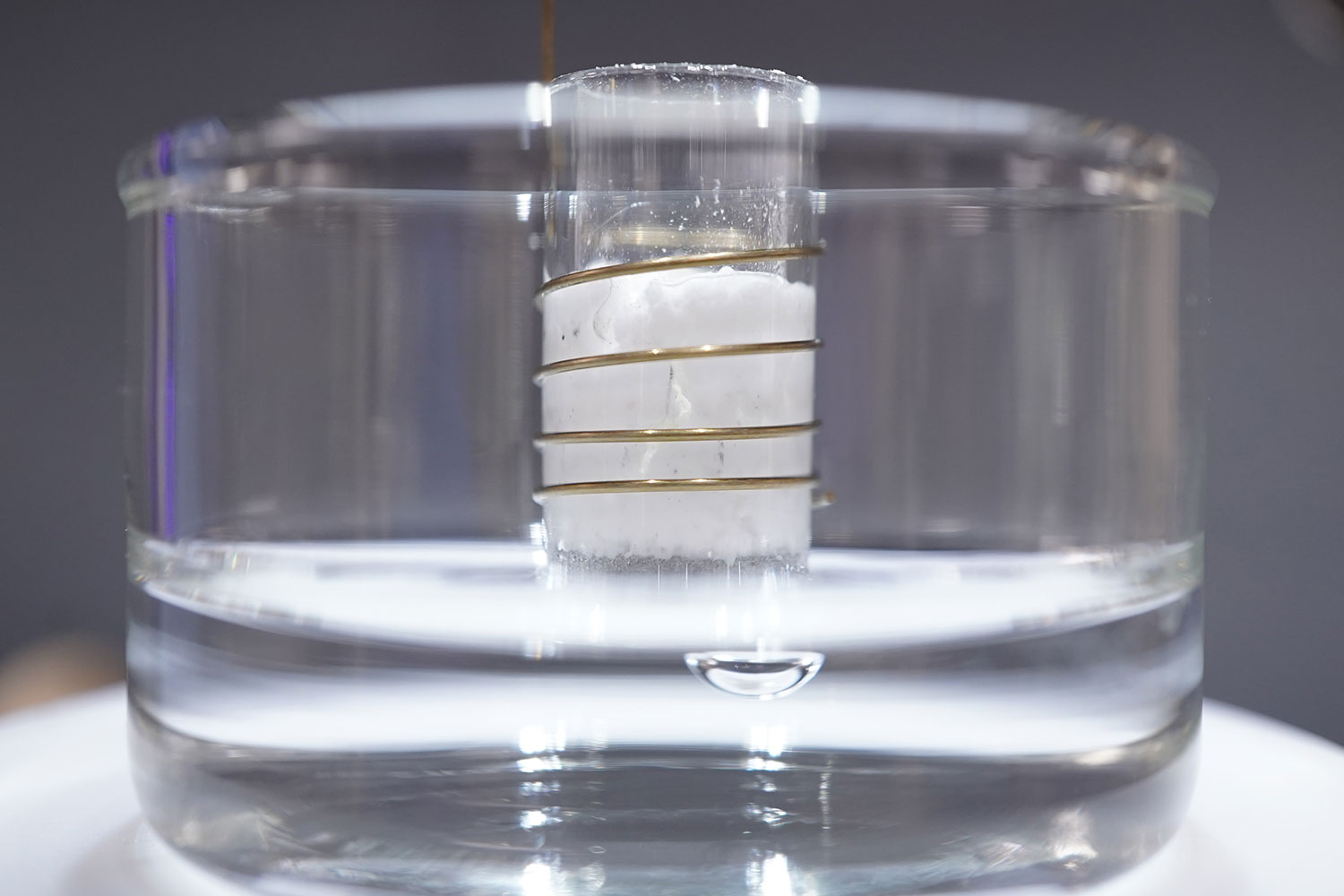
Typical compounding systems are designed for industrial-level production, with some processing hundreds of kilograms of material per hour. Especially when testing formulae with sensitive or expensive materials, these large-volume machines are far from ideal. Micro compounders provide a solution for this; with sample volumes as small as 2 mL, researchers can minimize waste, accelerate iteration, and lower costs. With the ability to process more than 25 formulations per day, micro compounders are also ideal for advancements in personalized medicine for patients with special needs. Micro compounding systems are also designed with scalability in mind, so any optimized formulations can be scaled to full-size systems once desired properties have been reached.
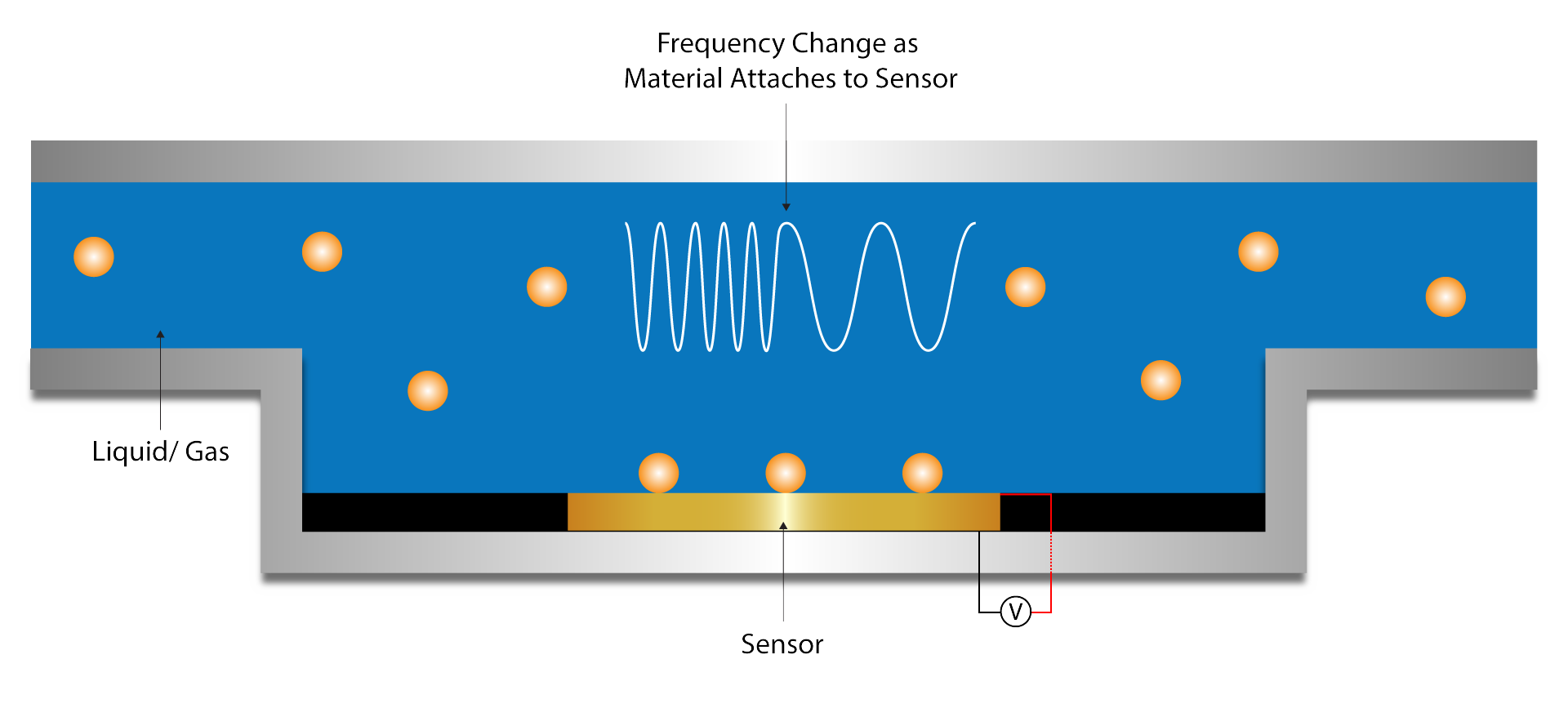
Biosensor Development
Solutions for Pharmaceuticals
Drug Characterization
Microsphere Cross Sectioning
Morphological Analysis and Impurity Detection
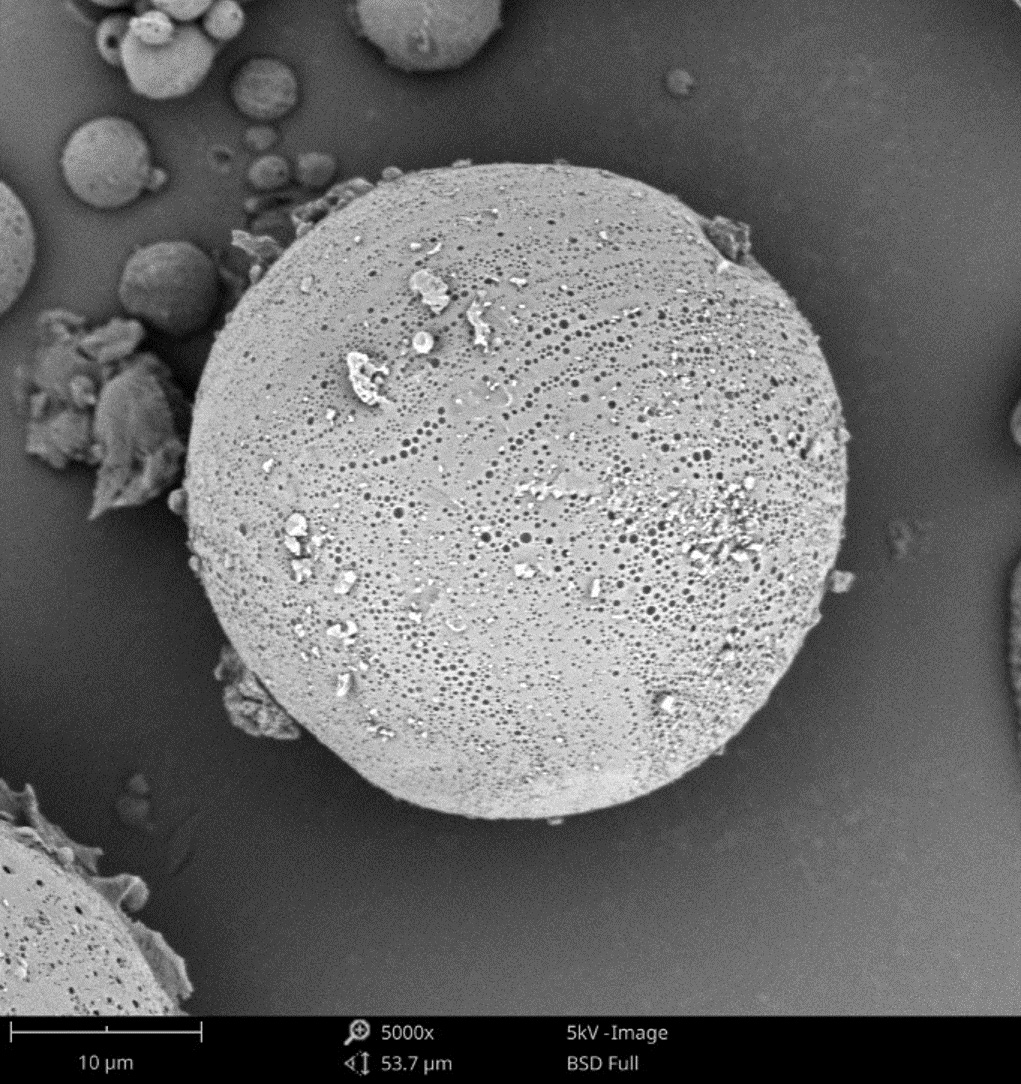
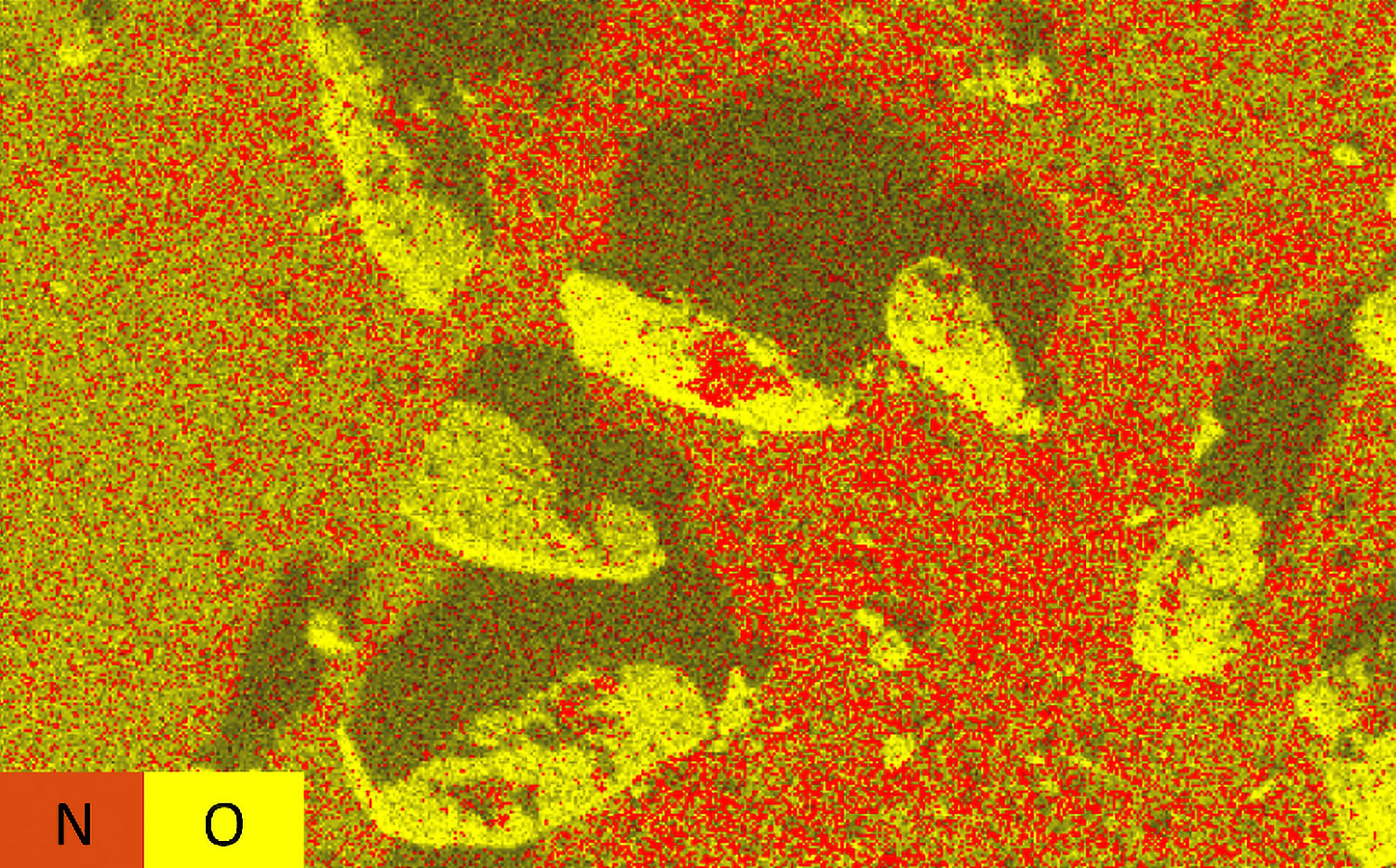
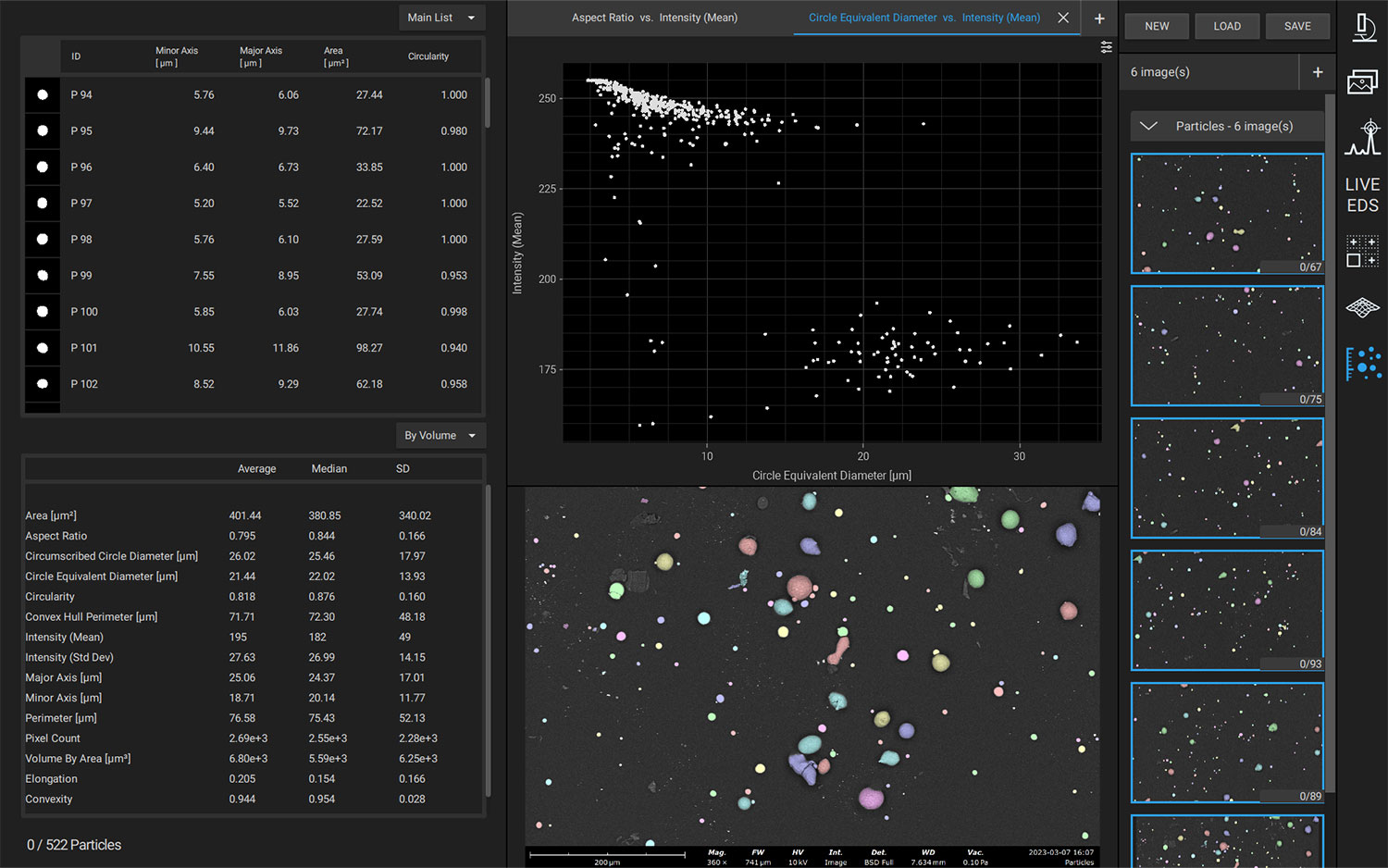
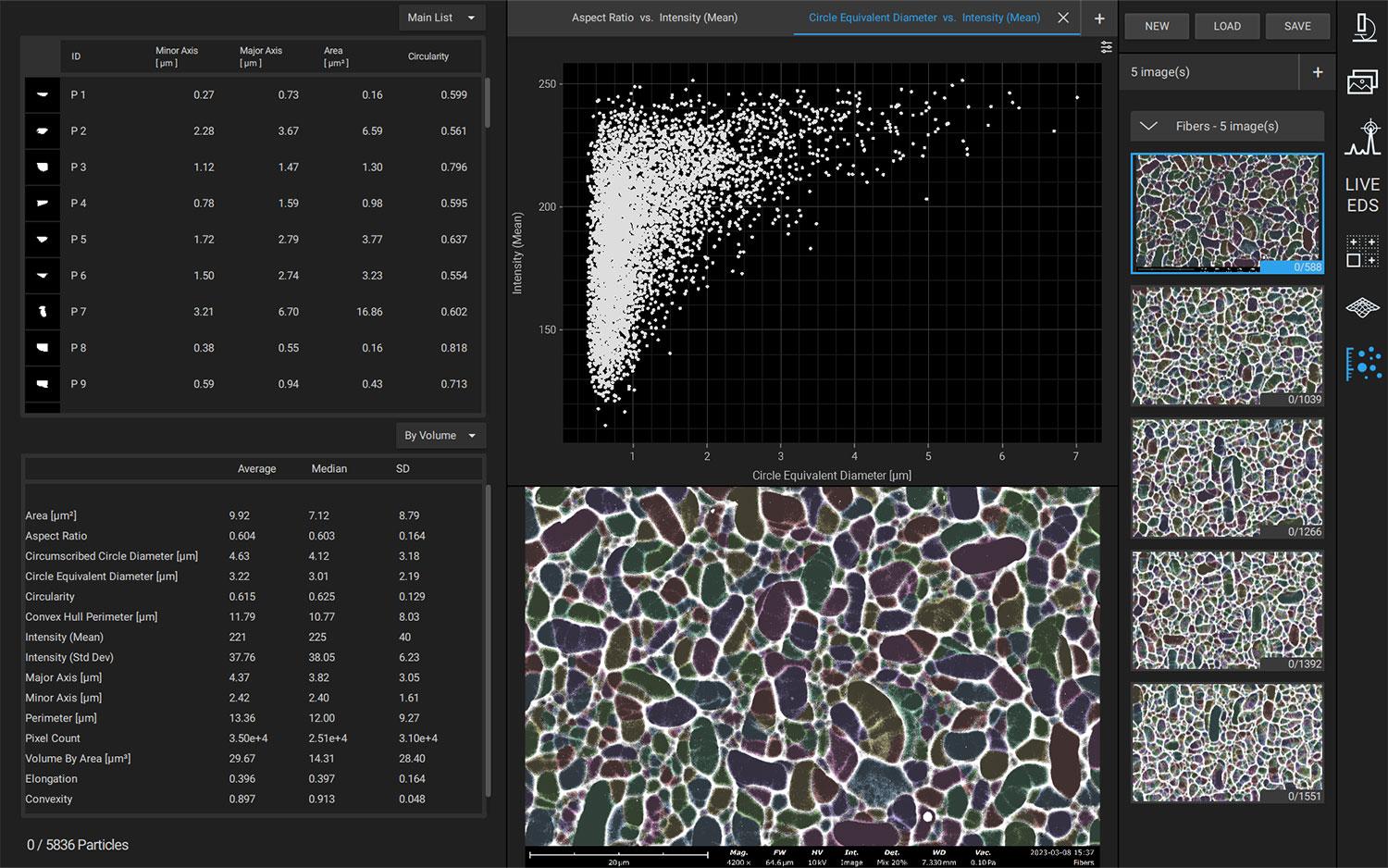
Nanoparticles are advantageous for drug delivery since smaller particles have improved absorption and solubility in the bloodstream. APIs can be chemically attached, encapsulated, adsorbed, or entrapped in nanoparticles, enabling computability with various API chemistries. The size, composition, and porosity of the final particles are all related to drug release efficacy. Also, any impurities or foreign particles introduce risks for therapeutic efficiency and patient safety. Therefore, understanding the morphologies of drug nanoparticles is essential for pharmaceutical development. When drugs are instead delivered via microneedle or other microscale method, morphological analysis is still crucial for ensuring quality.
Scanning electron microscopy (SEM) uses a focused electron beam to obtain high-resolution, nanoscale images of a sample. From these images, particle size distribution, porosity, and other morphological features can be measured. SEM can be combined with energy dispersive X-ray spectroscopy (EDS), which uses characteristic X-ray signals to identify elemental composition across a sample. This is particularly valuable for detecting impurities, which may appear similar to adjacent particles in standard SEM images. SEM-EDS is also capable of quantifying API distribution within excipient particles.
Automated SEM Workflows
Manual operation of SEM-EDS is adequate for many purposes; however, in applications where reproducible and bias-free results are prioritized, manual operation can be prone to errors. For pharmaceuticals, errors could lead to incorrect API dosage, therapeutic ineffectiveness, or even harm to the patient’s health.
Automation software for SEM-EDS allows for the construction and execution of customized recipes, improving reproducibility and reducing operator bias errors. Particle size, morphology, and elemental composition can be obtained across an entire sample with minimal intervention. Depending on the exact software used, custom reports can be generated after analysis to verify adherence to national (USP) or company-specific standards.
Wettability and Surface Tension Measurements
Interfacial Interaction Analysis
List of QCM-D Sensors for Drug-surface Interaction Studies |
||
| Plastic Packaging | Polypropylene (PP) Polyvinyl chloride (PVC) Polyethylene terephthalate (PET) Polymethylmethacrylate (PMMA) |
Polyethylene (PE) Low density PE (LDPE) High density PE (HDPE) Linear low density PE (LLDPE) |
| Glass Containers | Borosilicate glass |
Soda-lime glass |
| Bags | Cyclo olefin polymer (COP) |
Cyclo olefin co-polymer (COC) |
| Filter Materials | Polyvinylidene fluoride (PVDF) Polytetrafluoroethylene (PTFE) Polycarbonate (PC) |
Polyethersulfone (PES) Polyethylene terephthalate glycol-modified (PET-G) |
| Pre-Filled Syringes | PDMS (Silicone oil) | |
| Other Relevant Materials | Polystyrene Cellulose Stainless steel L605 SS2343 (Similar to US Standard 316) Ethylene-vinyl Acetate (EVA) |
Nylon Polyurethane Cellulose Acetate Polyacrylonitrile (PAN) |
Table 1. List of QCM-D sensors for drug-surface interaction studies
Solutions for Pharmaceuticals
Drug Delivery
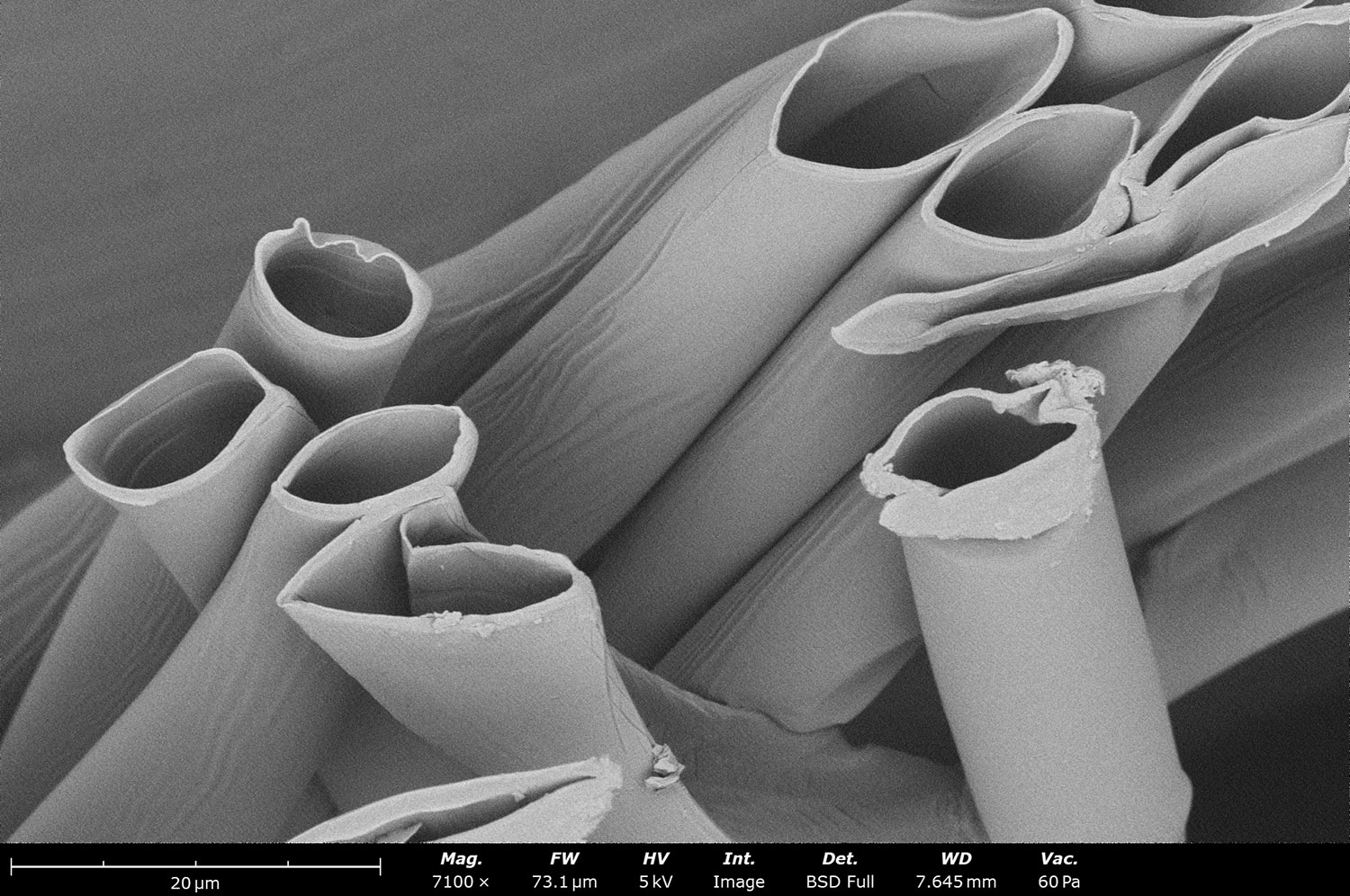
Nanofiber / Nanoparticle Drug Encapsulation
The benefits of targeted drug delivery include increased drug effectiveness, decreased toxicity, and protection of surrounding healthy tissue, among others. To achieve this, drugs must be incorporated into a material that is biocompatible to its target in the body, and they must degrade at the right time to expose the APIs. This can prove to be challenging, especially for complex, multi-layered tissues.
Electrospinning and electrospraying are techniques that produce nanofibers and nanoparticles, respectively. This is done by applying a high voltage between a solution emitter (typically a needle) and a collector; the electrohydrodynamic effects triggered by this voltage drop will drive fibers or particles from the emitter and onto the collector. Depending on material properties and instrument settings, different morphologies can be achieved, including fiber-particle composites made with blend, dual, or coaxial electrospinning. Particles, including APIs or cells, can be incorporated into electrospun scaffolds for targeted drug delivery to external and internal wounds. Oral, ocular, and stimuli-responsive drug delivery systems can all be fabricated with electrospinning.
Solutions for Pharmaceuticals
References:
-
- Chen, Q.; Tang, W.; Wang, D.; Wu, X.; Li, N.; Liu, F. Amplified QCM-D Biosensor for Protein Based on Aptamer-Functionalized Gold Nanoparticles. Biosensors and Bioelectronics 2010, 26 (2), 575–579. https://doi.org/10.1016/j.bios.2010.07.034. ↩︎
- Ma, Y.; Liang, J.; Zheng, J.; Wang, Y.; Ashraf, M.; Srinivasan, C. Significance of Cryogenic Broad Ion Beam Milling in Evaluating Microstructures of PLGA-Based Drug Products. Microscopy and Microanalysis 2021, 27 (S1), 90–91. https://doi.org/10.1017/s1431927621000945. ↩︎
- Li, Y.; Shi, J.; Zhang, X.; Ji, M.; Ni, Y.; Han, R.; Li, Z.; Xiong, Y.; Tu, J.; He, D.; Sun, C. Exploration of Surface Tension Measurement Methods for Pharmaceutical Excipients. International Journal of Pharmaceutics 2024, 123848. https://doi.org/10.1016/j.ijpharm.2024.123848. ↩︎
- Gagliardi, M.; Colagiorgio, L.; Cecchini, M. A Fast and Reliable Method Based on QCM-D Instrumentation for the Screening of Nanoparticle/Blood Protein Interactions. Biosensors 2023, 13 (6), 607. https://doi.org/10.3390/bios13060607. ↩︎
- Suthar, J.; Prieto-Simon, B.; Williams, G. R.; Guldin, S. Dual-Mode and Label-Free Detection of Exosomes from Plasma Using an Electrochemical Quartz Crystal Microbalance with Dissipation Monitoring. Analytical Chemistry 2022, 94 (5), 2465–2475. https://doi.org/10.1021/acs.analchem.1c04282. ↩︎