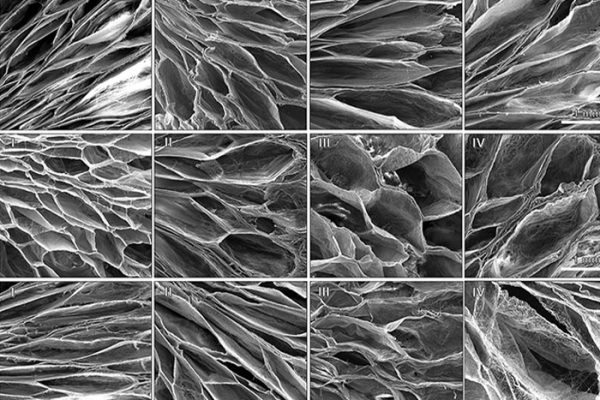
Glioblastoma (GBM) remains a highly aggressive brain cancer with poor prognosis, in part due to the limitations of systemic therapies crossing the blood–brain barrier and the recurrence of tumors post-resection. Interstitial drug delivery offers a promising alternative, yet current approaches often lack optimal control over therapeutic release kinetics. To address this, we developed electrospun nanofibrous scaffolds composed of acetalated dextran (Ace-DEX), a biodegradable polymer with tunable degradation and pH sensitivity, capable of modulating drug release rates. By engineering scaffolds with fast, medium, and slow degradation profiles, we achieved daily paclitaxel release rates of 14.1%, 2.9%, and 1.2%, respectively.
In orthotopic glioblastoma mouse models, we found that drug release rate significantly impacted therapeutic outcomes and combining fast and slow-release scaffolds resulted in 78% long-term survival, compared to only 20% with medium-release scaffolds delivering the same total dose. Beyond chemotherapy, we further explored localized immunotherapy using Ace-DEX scaffolds to deliver a TLR7/8 agonist, post-resection. This approach amplified immune activation in the brain and cervical lymph nodes, cleared residual tumor, improved survival, and conferred protection against tumor rechallenge. To streamline scaffold development, we established a machine learning workflow using Gaussian process regression (GPR) to accurately predict in vitro drug release kinetics from 30 Ace-DEX electrospun formulations. This predictive, drug-agnostic model accelerates preclinical testing and supports broader clinical translation. Together, these results highlight Ace-DEX nanofiber scaffolds as a versatile and powerful platform for controlled local delivery of chemo- and immunotherapies, with strong potential to improve GBM treatment outcomes.