Electrospun Nanofiber Scaffolding for Blood Vessels
One of the main goals of electrospun fibrous scaffolding is to completely biomimic the extracellular matrix (ECM) environment. This includes incorporating cells and sustaining their proliferation, having fiber orientation according to the structure being mimicked, allowing degradation of the biomaterial and production of new natural ECM by the seeded cells, along with completely resembling mechanical properties. Some commonly researched tissues where electrospun scaffolds are employed as this solution include tendons, heart valves, and vascular tissue. Typical materials for generating these grafting samples include the use of natural polymers like collagen, gelatin, and hyaluronic acid, among others.
Naturally, derived materials have the potential advantage of biological recognition, which may positively support cell adhesion and function. However, there are a number of drawbacks of using natural polymers. Some of them include rapid degradation in vitro and in vivo, low mechanical properties, high hydrophilicity and high variability between batches. Therefore, biomimicking the ECM environment with the use of natural polymers is not an easy task. For this reason, the use of synthetic polymers and blends of natural-synthetic polymers have been commonly employed in tissue engineering and regenerative medicine applications.
Biomimetic Properties of Polycaprolactone as Blood Vessel Scaffolds
One example of a broadly used synthetic polymer, polycaprolactone (PCL), has been used to create hollow tubular shaped scaffolds to biomimic vascular blood vessels. The inner diameter size of these PCL tubular scaffolds will depend on the desired blood vessel tissue, but they typically range from 1 to 5 mm. In terms of recreating the ECM structure of coronary arteries, a three-layered structure need to be engineered. By selecting an appropriate solvent with the right boiling point, the morphological and mechanical structure (fiber diameter, porosity, and mechanical properties) can be tailored to biomimic this type of tissue. For example, while the use of acetone can create a dense fiber to mimic the tunica intima layer, a higher volatile solvent like dichloromethane, can create flexible and large diameter porous fibers that can recapitulate the tunica media layer of the coronary arteries. The images below shows PCL fibers made with acetone and dichloromethane and typical tubular scaffolding material.
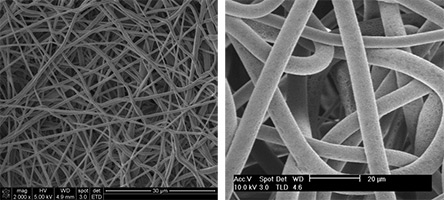

The use of the Fluidnatek® electrospinning equipment not only allows the use of different solvents during the electrospinning process, it also offers the ability to create tubular shaped scaffolds by employing a mandrel that can go up to 3,000 rpms. One crucial feature in scaffolds for tissue engineering and regenerative medicine applications is the creation of a uniform and homogeneous thicknesses. The Fluidnatek® equipment can be implemented with a programmable feature to control all process parameters as a function of time.
Delamination Challenges with Electrospun Medical Grade Tissue Structures
When multiple layers made with different polymer solutions are needed, researchers usually encounter delamination problems between these layers. The Fluidnatek® electrospinning equipment can be implemented with a feature to automatically control the Z-axis through a touchscreen. Although a completely dry product is usually desired, by decreasing the distance between the collector and emitter, the first and second layer can be physically connected by not allowing the solvent to completely evaporate during the spinning process. Remaining solvents are usually removed by vacuum drying the samples prior to in vitro or in vivo employment.
