
The rapid spread of coronavirus, COVID-19, is currently the most concerning matter worldwide. The outbreak has been declared a pandemic by the World Health Organization (WHO). The U.S. Food and drug administration (FDA), National Institutes of Health (NIH) and several other government organizations are reducing bureaucratic obstacles to meet the immediate need for vaccine development and treatment. While scientists are rushing to find a cure for COVID-19, the focus is on preventing it from further spread. Based on our latest understanding the coronavirus spreads from person to person who are in close contact. When an infected person coughs or sneezes, the respiratory droplets containing the virus can infect other people in close proximity (up to 6 feet).
Another source of transmission is likely through the contact of hard surfaces (doorknobs, railings, elevator switches, etc.) contaminated with the virus, making its way into the human body through the eyes, nose or mouth (hence the recommendation from the Center for Disease Control and Prevention (CDC) to avoid touching your face). These viruses have been found to remain active and infectious on hard surfaces for several days (Ref. # 1-3). The virus can be further transmitted to other surfaces continuing the spread.
Use of surgical masks and other PPE, frequent washing of hands with soap and water, and regular cleaning of hard surfaces has been recommended by the CDC to combat the spread of novel coronavirus.[Figure 1]
While research is underway to understand the exact nature of this virus, and evaluate the significance of transmission through hard surfaces, there are several reports suggesting that the structure of Novel coronavirus SARS-CoV-2 is one of the easiest virus types to be destroyed by detergents/soap and certain disinfectants [4-5].

Fig 1: Handwashing with soap to remove viruses
The anatomy of SARS-CoV-2 consists of a nucleocapsid core of single stranded RNA encapsulated in a spherical envelope made of lipid and membrane proteins, and club shaped spike proteins studded in the in lipid envelop [6-7, Figure 2]. The spike proteins help the virus attach to and infect human cells. The protective lipid coatings of these viruses are the easiest type to deactivate in contrast with many gastrointestinal viruses like norovirus which have a tough protein shell called a capsid.
Some products that can burst this lipid envelop are soap (usually composed of surface active agents called surfactants), alcohol-based disinfectants, bleach and other potent oxidizers. The U.S. Environmental protection Agency has released a list of EPA-approved disinfectant products that have qualified for use against SARS-CoV-2 virus.
The schematics in Fig 2 represent the proposed mechanism of how soap works on these viruses; when a virus contaminated surface is washed with soap and water, the soap molecules surround the virus. The hydrophobic tails of the soap molecules, that prefer the oil or lipid phase, penetrate to the lipid envelop, and rupture this layer. The resulting pieces of the destroyed virus are washed away in water as small micelles [8].
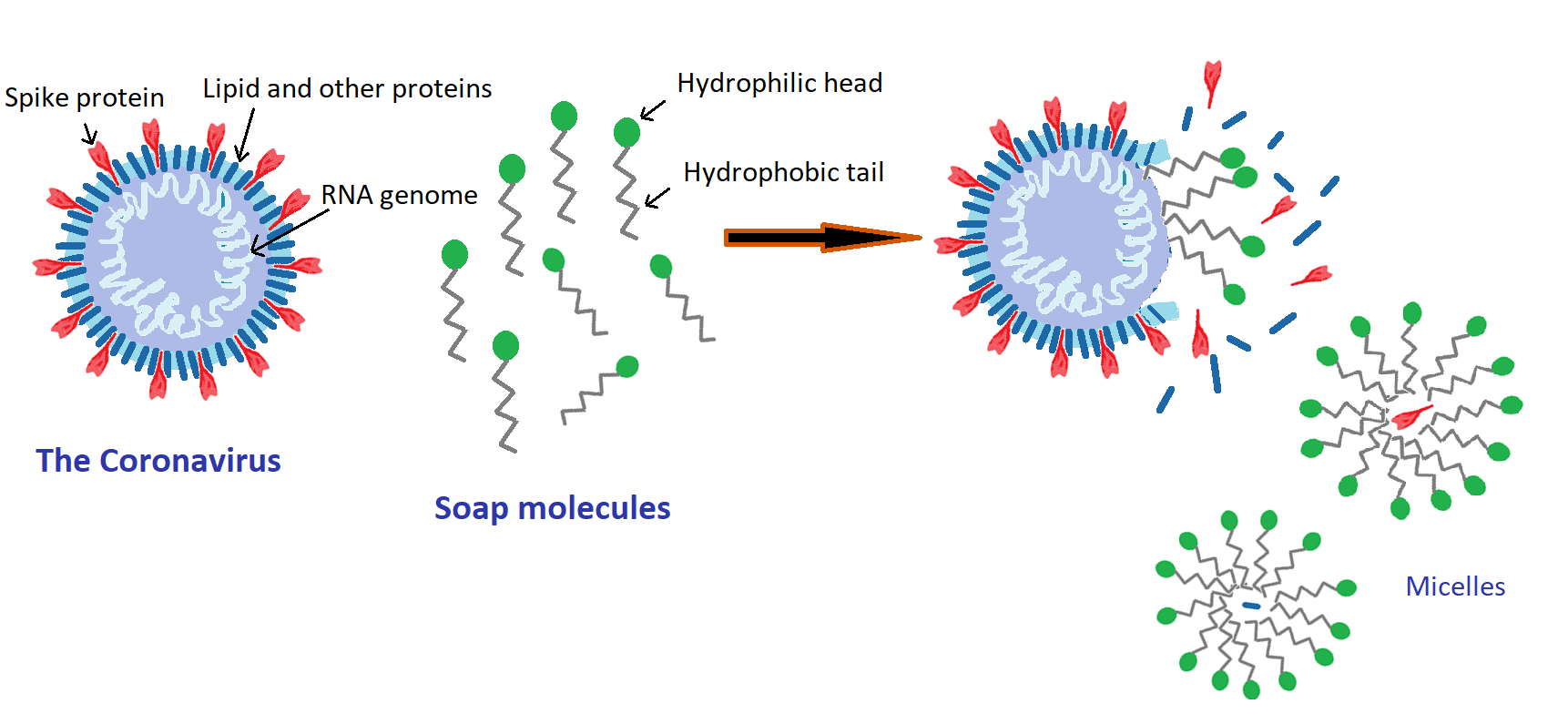
Fig 2: The proposed mechanism by which soap interacts with Coronavirus.
Analyzing how effectively a detergent cleans a surface is important and requires efficient tools. Quartz Crystal Microbalance with Dissipation (QCM-D) is a sensitive technique for analyzing nano-scale surface phenomena in real-time. It has been successfully utilized for real-time monitoring of mass removal (lipid, fat, grease, biofilms, etc.) from hard surfaces.
In a typical QCM-D experiment, a sensor with the desired coating to be tested is mounted in the flow module, which is then placed on the temperature-controlled measurement chamber. The sensor is excited by applying a suitable drive voltage, and two parameters; frequency shift ∆F and energy dissipation ∆D of the oscillating sensor are simultaneously monitored in real time. The frequency shift ∆F relates to the mass change on the surface while the dissipation change, ∆D provides information on the viscoelastic properties of the adhered mass.

Fig 3: The QSense Pro instrument (Biolin Scientific)
For analyzing mass removal by surfactants or detergents, the sensor is first coated with the material under evaluation. The detergent solution is then flown over the sensor surface, and the change in mass is monitored by measuring the ∆F and ∆D in a time resolved manner. An example of such a study is presented in fig 4 (data is obtained from Biolin Scientific).
Lipid removal by surfactants
In this example, removal of a lipid known as Triolein, by the non-ionic surfactant Triton X-100 is analyzed using QCM-D. As a first approximation, this lipid layer may relate to a virus’ outer shell and information may be gleaned about how it could be possible to remove this from a surface. Triolein was spin-coated onto a gold QCM-D sensor to produce a model surface. The interaction and removal efficiency using Triton X was subsequently analyzed as a function of temperature and concentration.
The results indicate Triton X-100 initially caused swelling of triolein at all concentrations. (Fig. 4). At concentrations below the CMC Triton X-100 either adsorbed (0.1 x CMC) or partially removed the layer (0.9 x CMC). However, at concentrations above the CMC (10 x CMC) almost all of the layer was removed (Fig. 3). The rate of mass removal was affected by temperature, as seen in the right plot of figure 3 below. with faster removal rates at higher temperature.
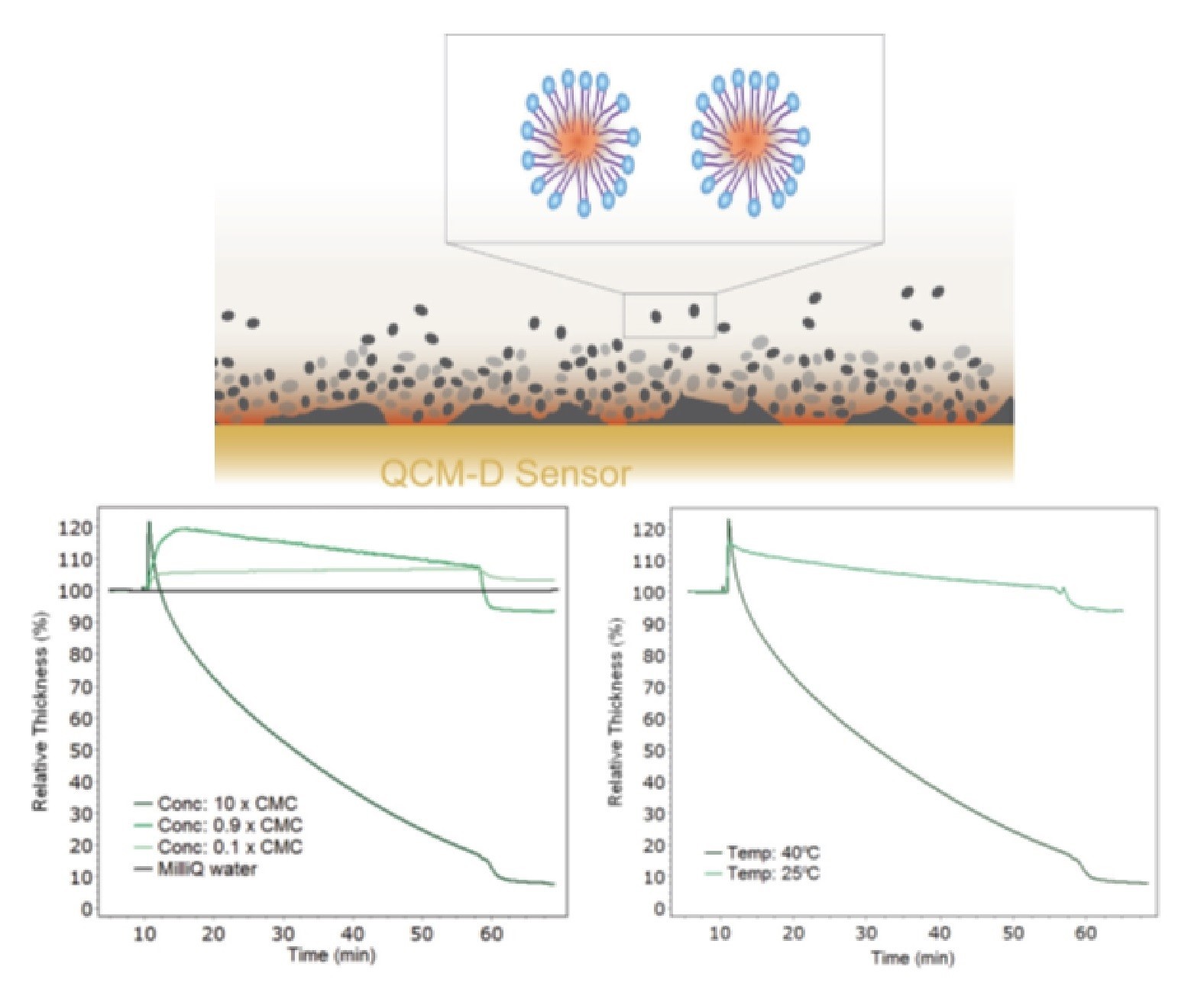
Fig 4: Changes in 40 nm thick lipid film upon exposure to TritonX-100 surfactant (10 min) and subsequent water rinse (58 min) at 40 °C (left plot). The 100%Thickness refers to the as prepared thickness of the triolein film. The temperature dependence is shown in the right plot.
Response of live bacterial cells to surfactant and Lipopolysaccharide
In another example, Fatisson et al. [9] used QCM-D for real-time in situ detection of cytoskeletal changes in live primary endothelial cells in response to cytomorphic agents; namely, the surfactant Triton-X 100 (TX-100) and bacterial lipopolysaccharide (LPS). The coating of bovine aortic endothelial cells (BAEC) onto a silica coated sensor was achieved by first modifying the surface with poly-l-lysine (PLL) polymer followed by incubating it in a suspension of 5 × 104 BAEC for 24 h (37 ◦C, 5% CO2), prior to being mounted into a QSense flow chamber. The cell coated sensor was then equilibrated in warm cell culture medium (37 ◦C). Finally, the cells were challenged with the above-mentioned surfactants in cell culture medium and the real-time response (cytoskeletal changes) were monitored by QCM-D [9].
Cell response to cytomorphic agents are related to the cell’s morphological changes i.e. cell shrinkage and lysis due to TX-100 effect, as well as cell rounding induced by LPS, resulting from different mechanical and/or biochemical pathways. The results show that these morphological changes can be detected and characterized by QCM-D. Furthermore, the cell toxicity can also be predicted since cytotoxic agents induce different responses in QCM-D signals.
By adopting a similar approach, it could be possible to use QCM-D for analyzing the effect of cleaning and disinfectant products onto SARS-CoV-2 coated surfaces under various conditions. There are a variety of hard coatings including metals, metal oxides, glasses, steels, and polymers ready to be used in such studies to mimic real world surfaces. The sensors can be obtained from Nanoscience Instruments. The Nanoscience Analytical (ISO 9001:2015 Certified), a sister company of Nanoscience Instruments also offers QCM-D measurements and other analytical services.
References:
- Journal of Hospital Infection 2020, DOI: 10.1016/j.jhin.2020.01.022
- MedRxiv 2020, DOI: 10.1101/2020.03.09.20033217
- New England Journal of Medicine (2020, DOI: 10.1056/NEJMc2004973
- https://www.vox.com/science-and-health/2020/3/11/21173187/coronavirus-covid-19-hand-washing-sanitizer-compared-soap-is-dope
- https://cen.acs.org/biological-chemistry/infectious-disease/How-we-know-disinfectants-should-kill-the-COVID-19-coronavirus/98/web/2020/03
- https://www.scientificamerican.com/article/how-coronaviruses-cause-infection-from-colds-to-deadly-pneumonia1/
- https://cen.acs.org/analytical-chemistry/structural-biology/Structure-novel-coronavirus-spike-protein/98/i8
- https://www.nytimes.com/2020/03/13/health/soap-coronavirus-handwashing-germs.html
- J. Fatisson et al. Biosensors and Bioelectronics 26 (2011) 3207–3212 https://www.sciencedirect.com/science/article/pii/S0956566310008626
