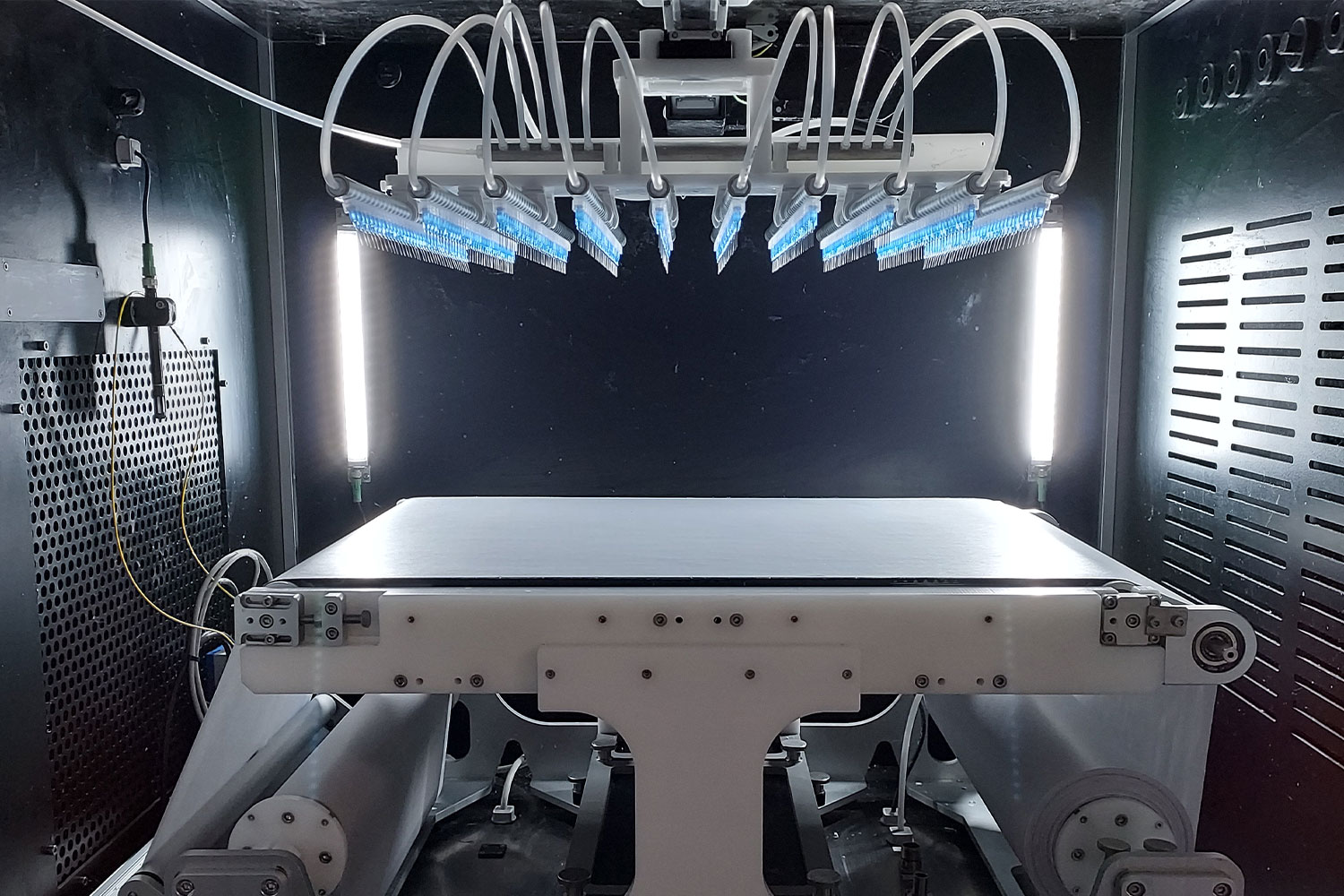
Electrospinning & Electrospraying System
Fluidnatek LE-500 BioDevice
The Fluidnatek LE-500 BioDevice is designed to coat non-symmetrical and complex structures like medical implants and biomedical products. The built-in robotic arm provides the flexibility needed to generate precise movements that result in uniform, homogeneous coatings of electrospun fibers or electrosprayed particles to enhance biocompatibility, support drug elution, or customize physicochemical properties.
Maximize Coating Production
Multidirectional sample processing allows coating of any 3D medical implants and medical devices with complex geometries
Full Environmental Control
HEPA- filtered unit provides control over temperature (18 – 45 °C), relative humidity (10 – 80%), and airflow (90 – 180 m³/h)
Robotic Arm for Full Flexibility
Completely programable arm able to hold rotating platform or the needle-injector for fabrication on complex structures
Production Versatility
Process up to 8 Liters of solution with up to 370 needles to generate pilot-scale quantities of electrospun or electrosprayed material
Explore electrospinning for your application!
Our contract research team offers you access to latest in electrospinning, ideal for initial consultations, proof-of-concept experiments, feasibility studies, method development, and early-stage R&D.
The Only Electrospinning System with a Fully-Articulating Robot Arm to Apply Uniform Coatings to Medical Devices
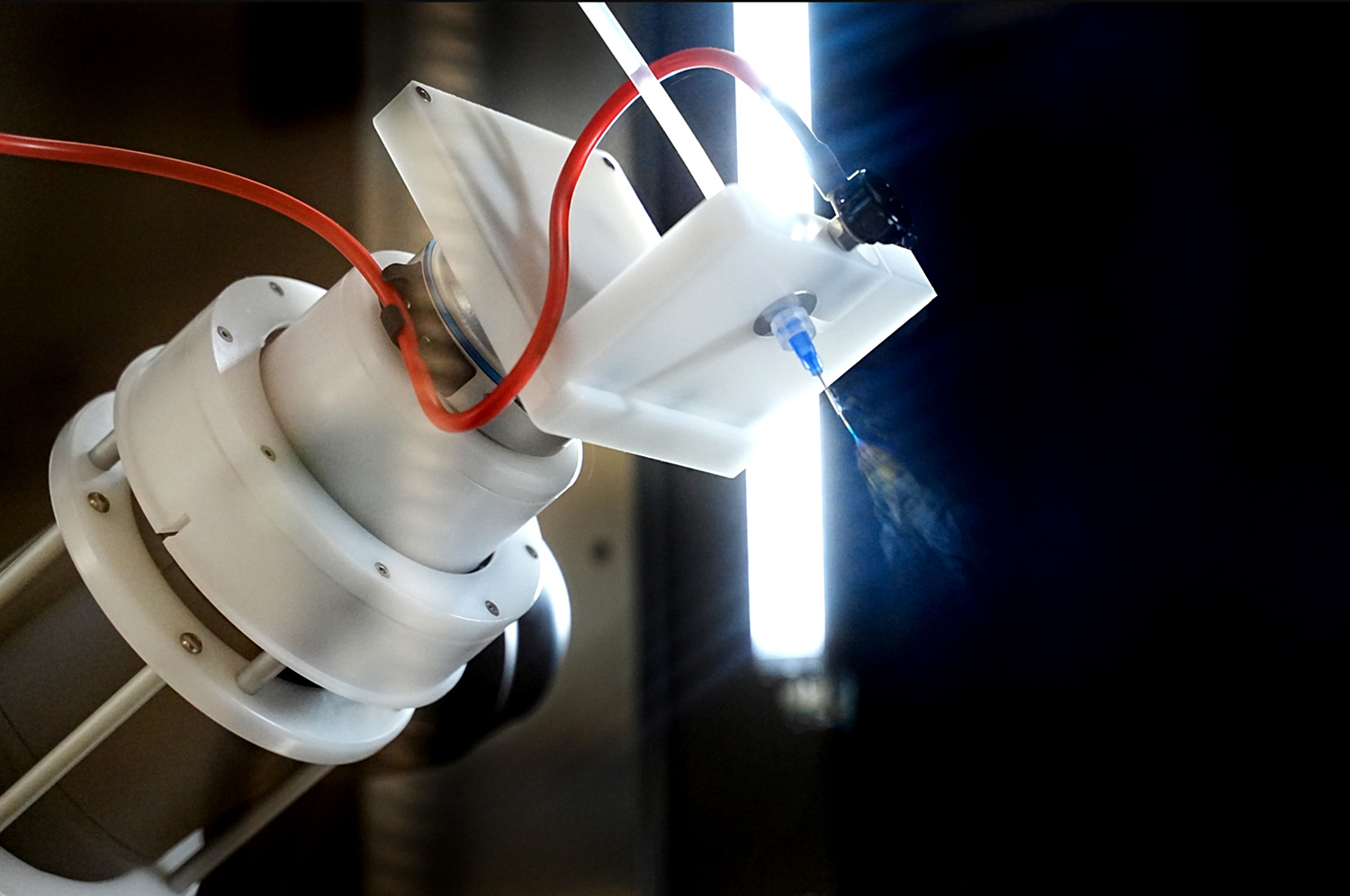
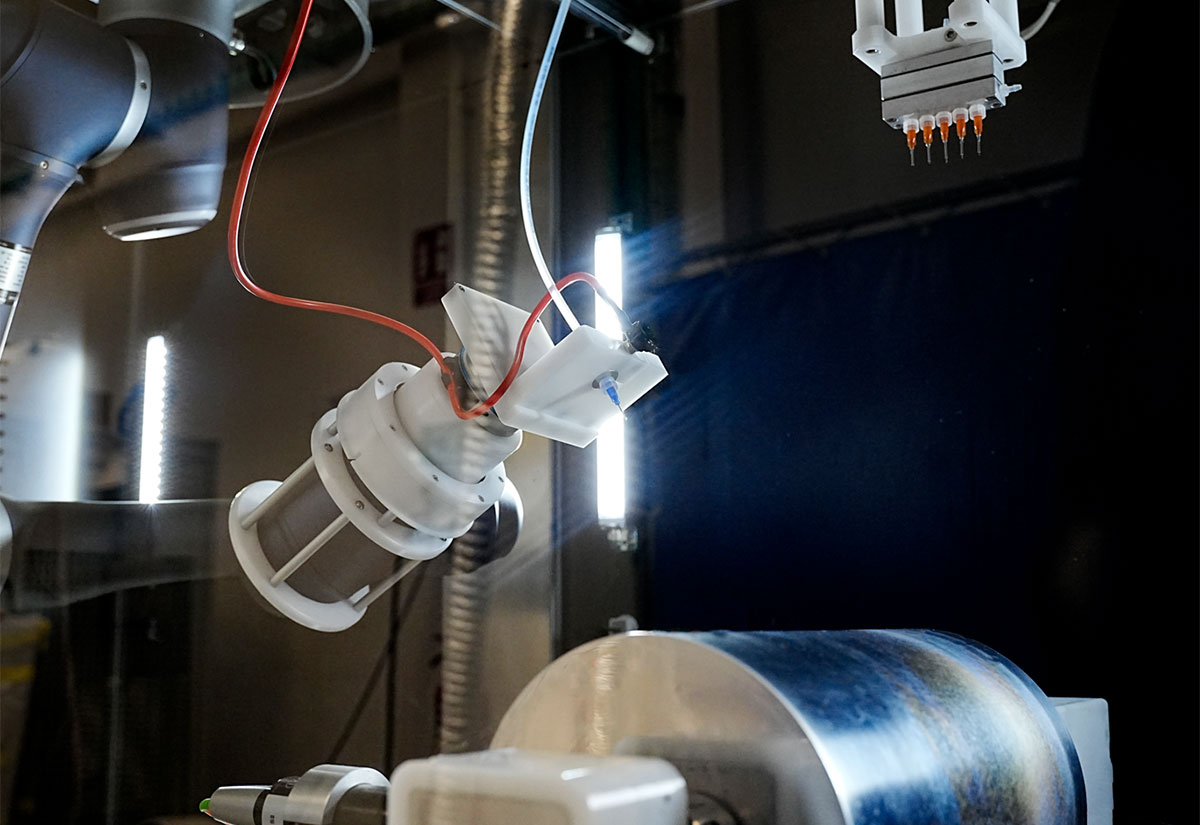
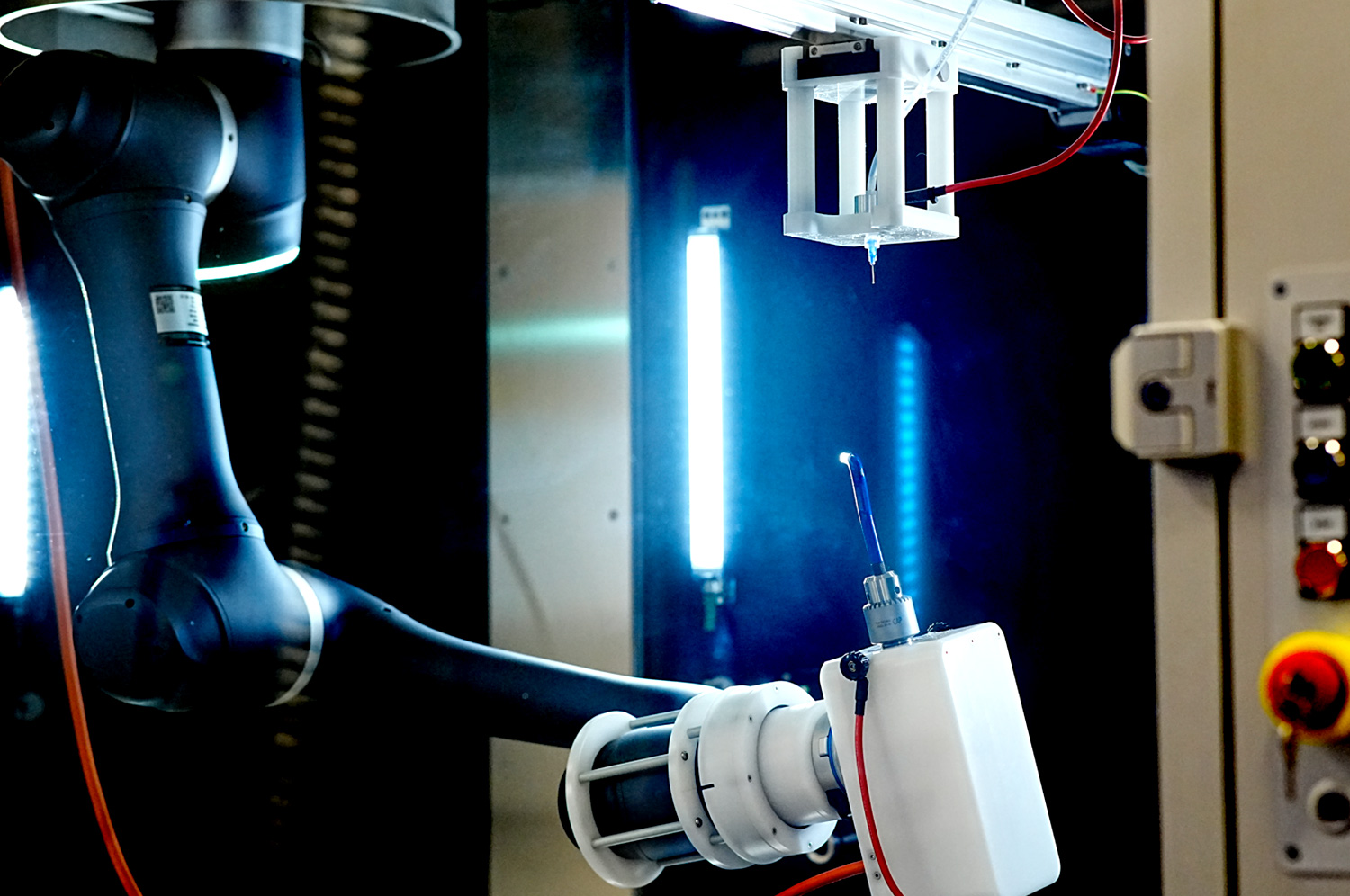
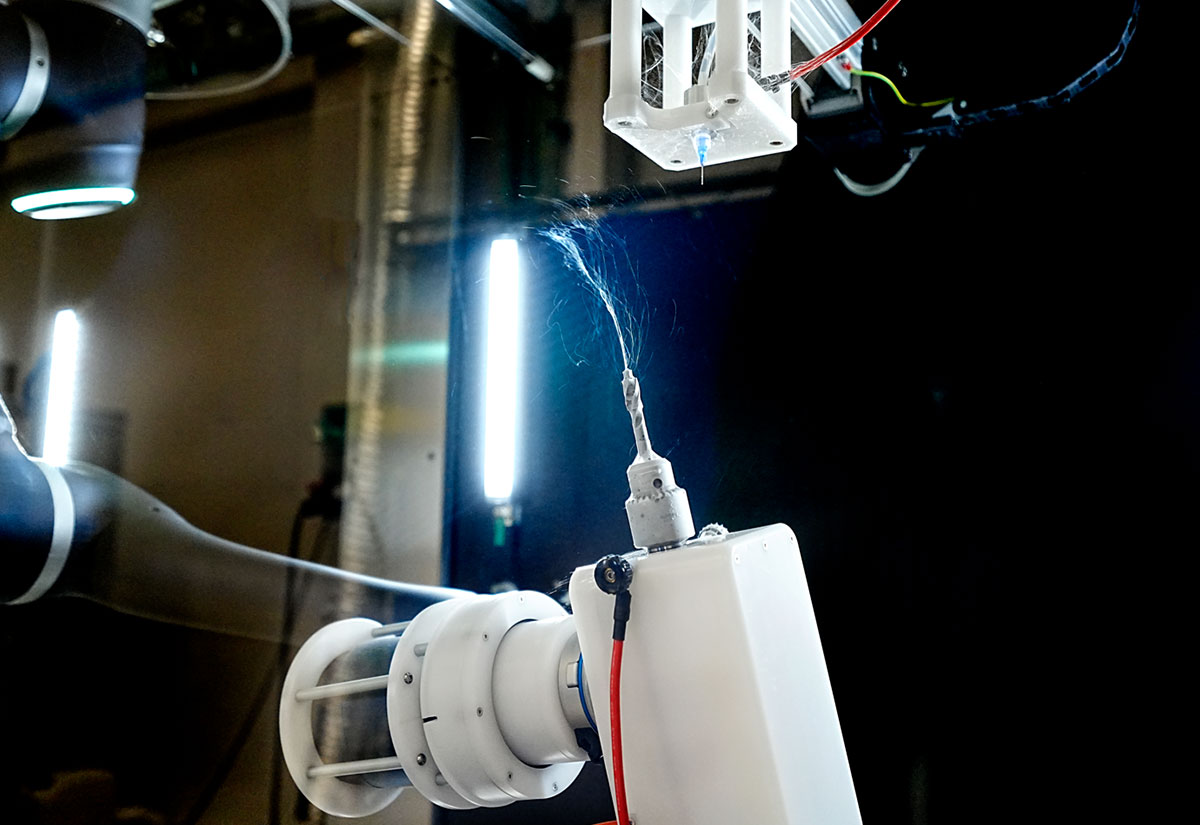
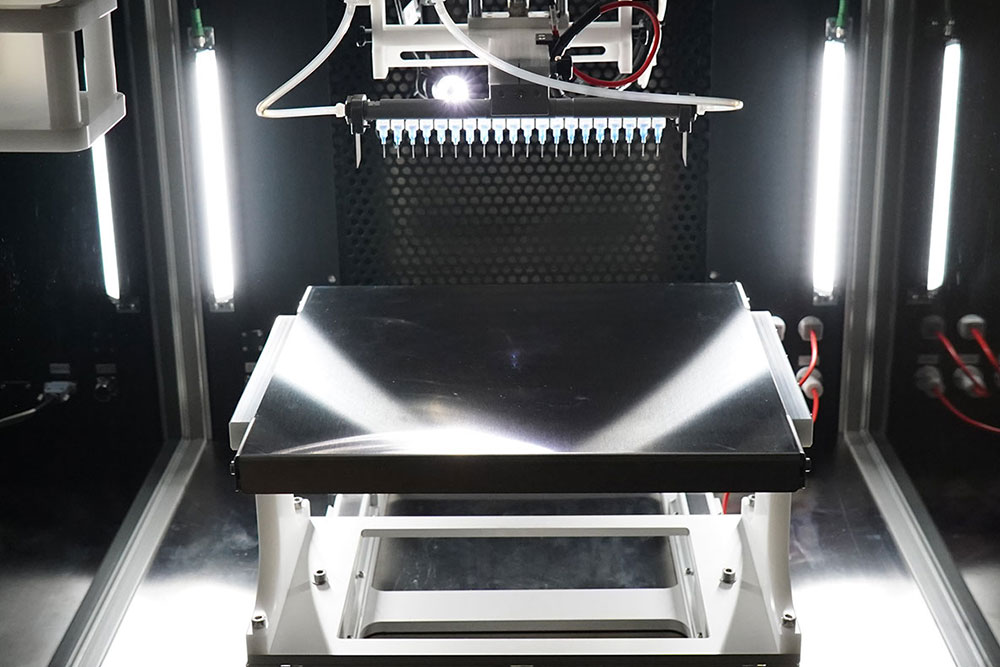
The LE-500 BioDevice is the first electrospinning machine with integration of a collaborative robot (CoBot) arm inside its chamber. This unique feature offers an unparalleled ability to manipulate the collector or emitter in 3D space, enabling multi-step processing and variable deposition angle to coat complex medical implants, medical devices, and 3D parts. This unit also offers all capabilities of the standard LE-500 machine, including full environmental control for humidity, temperature, air flow, and clean processing. In addition, users can employ up to 370 emitter nozzles to produce electrospun fibers or electrosprayed particles, making this a powerful tool for product commercialization. Multiple optional upgradable features to improve production can also be implemented at any time. This unit is capable of being ISO-13485 certified for medical devices and can operate at ISO-7 clean conditions.
Application Areas:
Fluidnatek LE-500 BioDevice
Product Features
Pilot-Scale Production Unit for Medical Products
The LE-500 BioDevice is designed for translational research and pre-clinical development of medical and biomedical products, offering users the ability to create full-scale prototypes and pilot-scale batches of electrospun or electrosprayed devices. With the flexibility to construct entirely electrospun or nanofiber-coated devices in a processing chamber that maintains ISO 7 cleanliness, it is an ideal solution for R&D teams working towards commercialization and regulatory approval.
Robotic Arm Option (CoBot)
An optional robotic arm provides a customizable method of applying uniform coatings to pre-fabricated objects or creating uniquely-shaped 3D structures out of electrospun and/or electrosprayed material. The programmable, fully articulating arm allows precision electrospinning at any angle you choose or can manipulate an object in 3D space for complex coatings. Each CoBot is designed specifically based on the customer’s needs and desired outcomes.
Process Validation
The LE-500 BioDevice can be provided with qualification documentation to support validation compliance, including Factory Acceptance Testing (FAT), Site Acceptance Testing (SAT), Installation Qualification (IQ), and Operational Qualification (OQ). These documents facilitate process validation and comply with standards such as Good Manufacturing Processes (GMP), Good Laboratory Practices (GLP), and Good Automated Manufacturing Practices (GAMP), meeting Food and Drug Administration (FDA) and International Organization for Standardization (ISO) requirements.
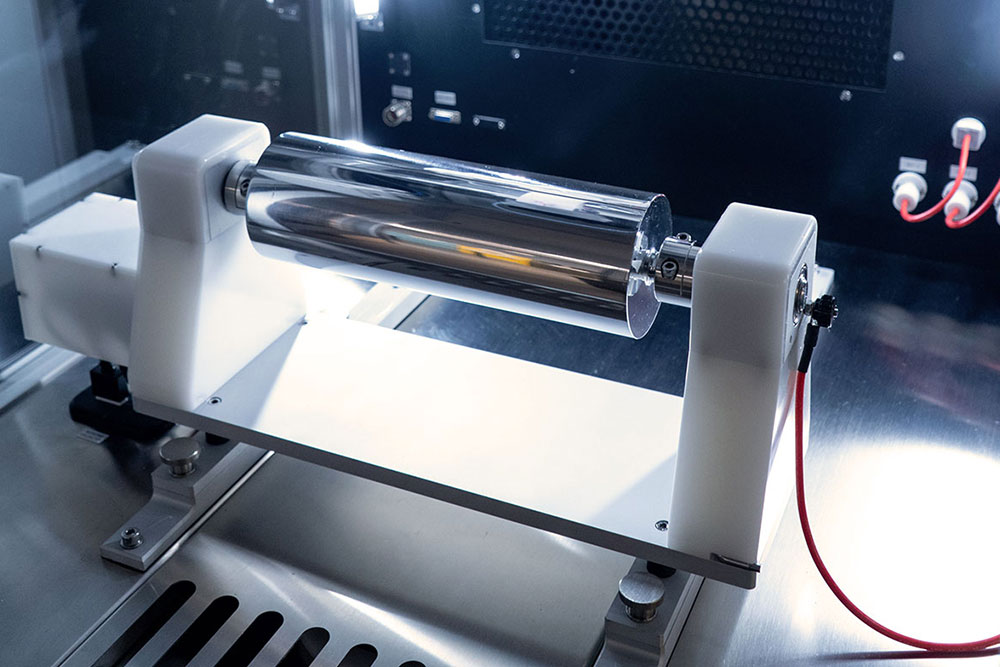
Rotating Collector Platform with Adaptable Collector Mount
The LE-500 BioDevice system has expanded options for rotating collectors, offering more flexibility than standard drum collectors. Specialized collector systems allow one-ended or double-ended mounting to accommodate a variety of mandrel diameters and non-symmetrical collectors like customized stents.
Versatile Solution Feeding
Offers versatile solution delivery options, including syringe pumps, peristaltic feeding with gravimetric control systems and stirring systems, and pressurized feeding systems. Three types of syringe pumps are available to suit different needs: single-channel pumps for typical low viscous solutions, high-pressure twin-channel pumps for precise dual-solution delivery, and high-pressure four-channel pumps for larger volumes and solutions with high viscosity. The peristaltic pump with a gravimetric system is the ideal solution feeding system for increased throughput processes with batches up to 8 liters.
Fluidnatek LE-500 BioDevice
Product Accessories
Environmental Control Unit (ECU)
The optional Environmental Control Unit ensures tight control of environmental conditions by regulating temperature (18 – 45 °C) and relative humidity (10 – 80%), with stability ranges of ±1°C and ±3%. This feature enables reproducibility in fiber and particle morphology, making it ideal for advanced applications that require stable environmental conditions. The ECU is the ultimate accessory for batch-to-batch consistency all year round.

The BioDevice can also be modified to incorporate an open-loop nitrogen environment, reducing oxygen concentration by introducing a continuous nitrogen supply. This feature is particularly beneficial when working with flammable or explosive solvents, as it enhances operational safety by minimizing the risk of combustion. The system automatically disables processing if the oxygen concentration exceeds a user-defined safety limit, ensuring a controlled and safe environment during sensitive electrospinning processes.
High Definition Process Data Hub (HDPDH)
The optional HDPDH provides real-time updates, monitoring, and control of the electrospinning unit with 20+ viewable process parameters. This powerful, unique Industry 4.0 software tool provides a detailed record of process stability over time. The Process Data Hub ensures process consistency and repeatability between batches and across platforms, providing smooth scalability to higher throughput. The Audit Trail and Batch Control modules can be included in the HDPDH software help support GMP, ISO, or other regulatory certifications for biomedical products.
Collector Variety
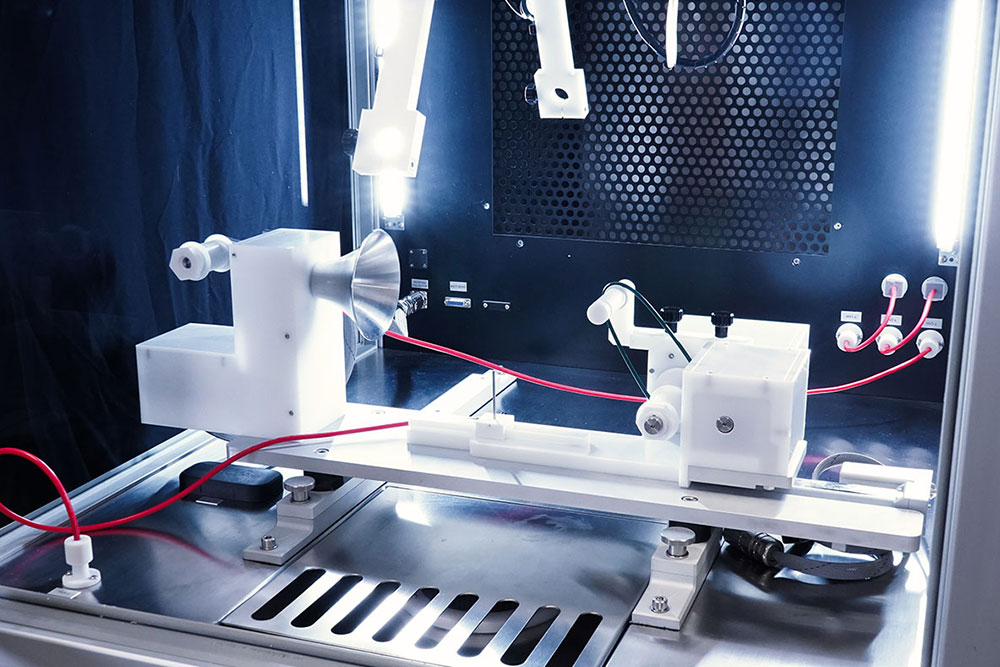
The system supports multiple plug & play collectors, such as flat plates, rotating drums, mandrels, and disk collectors. These options enable the creation of randomly oriented or aligned fibers, tubular structures, and large samples. For high-throughput production, collectors like roll-to-roll systems with conveyor belts are used to gather meters of material efficiently. Continuous yarns, composed of the same or multiple materials, can be collected using the electrospun fiber-yarn collection module.
Syringe Pump Options
The LE-500 BioDevice supports various syringe pump types, including single-channel, twin-channel, and four-channel syringe pumps. They can be used in single-phase, coaxial, and triaxial configurations. These pumps accommodate batch volumes ranging from 1 mL to 1,600 mL, providing flexibility for diverse experimental setups. Linear forces up to 4,400 N allow users to operate with high-viscosity solutions for the most challenging applications.
High Voltage Options
The BioDevice includes dual high-voltage power supplies for emitters and collectors, capable of generating up to +30 kV in the emitter and -30 kV in the collector. The emitter voltage can be increased to +50 kV with an optional upgrade for enhanced throughput and stability in demanding applications. An optional bipolar high-voltage power source up to ±30 kV is also available for when flexibility during production is needed. Bipolar capability allows for the reorganization of functional groups in the material at the molecular level, enabling easier optimization of sample properties like wettability and mechanical strength. All these configurations enhance process flexibility for complex material deposition, such as improved adhesion when coating different samples or substrates.
Advanced Emitter Options
A variety of emitters are available, including single-phase, coaxial, and triaxial configurations, as well as others tailored for specific applications like bipolar or multi-layered samples. The system supports up to 370 emitters in multi-emitter configurations for high-throughput production, gas-assisted processing, and it offers needleless slit injectors, capable of generating multiple jets along their length, for significantly increased production capacity. These options provide flexibility for producing complex nanostructures and scaling up manufacturing processes. Optional accessories like the gas-assisted electrospinning modules increase throughput and process stability. A solvent gas jacket system prevents tip drying for volatile solutions.
Motion and Positioning Controls
When a robotic arm is not in use, automated Y, and Z-axis motion systems allow precise control over emitter positioning and distance. These features improve sample consistency and enable the production of uniform coatings or complex structures; especially when operating with the roll-to-roll and rotating collectors.
Process Optimization Tools
Accessories such as a solvent-gas jacket system, gas-assisted electrospinning modules, and Taylor cone visualization systems enhance process stability, especially when working with volatile or high-viscosity solutions.
Additionally, the in-line area density metrology system provides real-time measurement and monitoring of deposition uniformity during roll-to-roll processing. To further enhance process control, Industry 4.0-oriented software features enable continuous real-time monitoring of all machine parameters, with updates every second. These capabilities allow users to remotely visualize the machine’s operation and conduct in-depth analyses of each batch, facilitating improved traceability and reproducibility.
For added standardization, a batch ID feature can be implemented to streamline process documentation. Furthermore, an optional Audit Trail upgrade logs all events occurring during machine operation, providing a comprehensive record that supports quality assurance and compliance requirements.
Fluidnatek LE-500 BioDevice
Electrospinning & Electrospraying Applications
Tissue Engineering
Electrospun nanofibers are used to create scaffolds for tissue engineering applications. These scaffolds mimic the extracellular matrix, providing a supportive environment for cell growth and tissue regeneration. They are prevalent in wound healing, bone regeneration, nerve regeneration, and more.
Bio Textiles
BioTextiles are fibrous materials used in healthcare and biomedical applications. Electrospinning offers a highly customizable method of fabricating biotextiles, allowing careful tuning of mechanical, chemical, and biological properties of the nanofibrous material. Electrospun biotextiles are typically biocompatible and can be fabricated from polymers or organic materials that are biodegradable or bioresorbable. Electrospun yarns can be loaded with active pharmaceutical ingredients (APIs) to create drug eluting sutures,
Medical Devices
Whether as coatings for prefabricated structures, active ingredient additives, or total device fabrication, medical devices implement electrospun nanofibers and particles for enhanced therapeutic effectiveness. These fibers and particles can allow for improved performance in mechanical properties, biological microenvironment control, cellular integration, and many more application-specific advantages.
Drug Delivery
Electrospun fibers and/or particles can be loaded with drugs or therapeutic agents and used as drug delivery systems. Precisely controlled electrospinning parameters allow the high surface area-to-volume ratio and tunable properties of nanomaterials, providing controlled release of drugs, making them suitable for targeted and sustained drug delivery.
Fluidnatek LE-500 BioDevice
Product Knowledgebase
Webinar
Advancing sustainable, animal component-free (ACF) materials for cell culture is critical…
Fabricating Nanofibers: Electrospinning Vs Blow Spinning
Nanofibers, with their high surface area-to-volume ratio, tunable porosity, and mechanical…
White Paper
Fabrication and Characterization of Electrospun Fibers
Polymer fibers are produced from synthetic or natural polymers and engineered to deliver a…