Regenerative Medicine Utilizing Electrospun Scaffolds for Wound Healing
Imagine a world where no organ donors are needed; a world where any type of tissue can be artificially created and function as compared to native tissue without rejection or scar formation. Scientists and engineers are currently working to achieve this goal through tissue engineering. There are multiple techniques that are employed to create scaffolding material to provide functional tissue and restore, maintain or improve tissues or organs. Among these, the electrospinning technique has gained popularity. The electrospinning technique has the ability to create scaffolding material at room temperature that will mimic the extracellular matrix (ECM) structure. It can create samples by co-electrospinning fibers and electrospraying cells at the same time, biomimic mechanical properties of different tissues, tailor fiber orientation, and post-process after sample formation. These qualities, among others, make electrospinning an excellent solution for tissue engineering and regenerative medicine applications.

Regenerative Medicine for Tissue Repair and Wound Healing
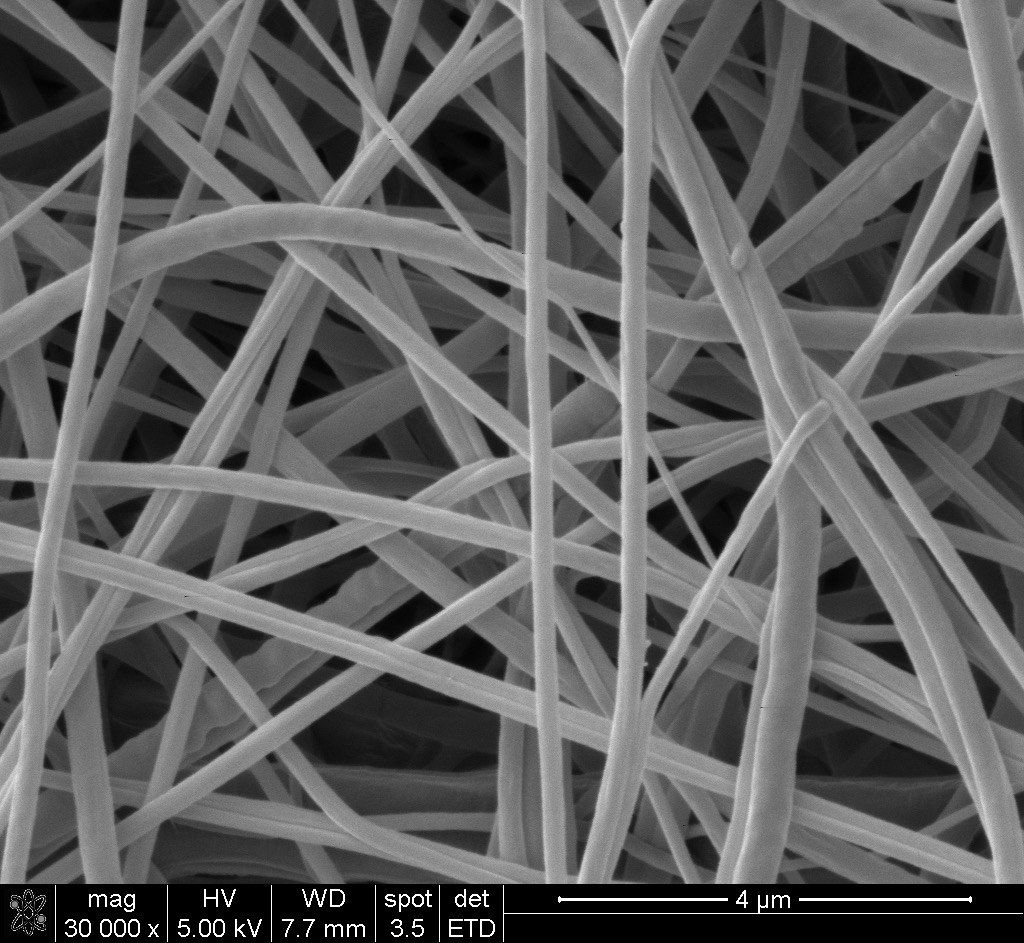
Regenerative medicine is a broad field that includes tissue engineering. It involves the repair and self-healing ability of the main body with the aid of an external material to recapitulate the natural environment. This can be done with an electrospun scaffolding material that resembles the in vivo environment, typically with the aid of a bioactive material. The concept behind regenerative medicine is to provide support and mechanical strength until the damaged tissue is regenerated. Depending on the selected biocompatible material, it can remain permanently in the body or be fully resorbed and replaced by the body’s own tissue.
In some cases, wounds are non-responsive to traditional therapies and dressings, therefore impeding the wound healing process. This is being addressed by the use of electrospun scaffolds. Researchers are engineering these scaffolds to completely mimic the in vivo environment of the targeted tissue. For example, Renovoderm employs the Phoenix Wound Matrix, a non-woven FDA approved electrospun graft made with the Fluidnatek® technology, to treat different types of wounds. Some examples include partial and full-thickness wounds, pressure ulcers, venous ulcers, diabetic ulcers, chronic vascular ulcers, surgical wounds, trauma wounds, and draining wounds. The healing process with the electrospun graft is depicted in the image below.
Learn more about Phoenix Wound Matrix Research.
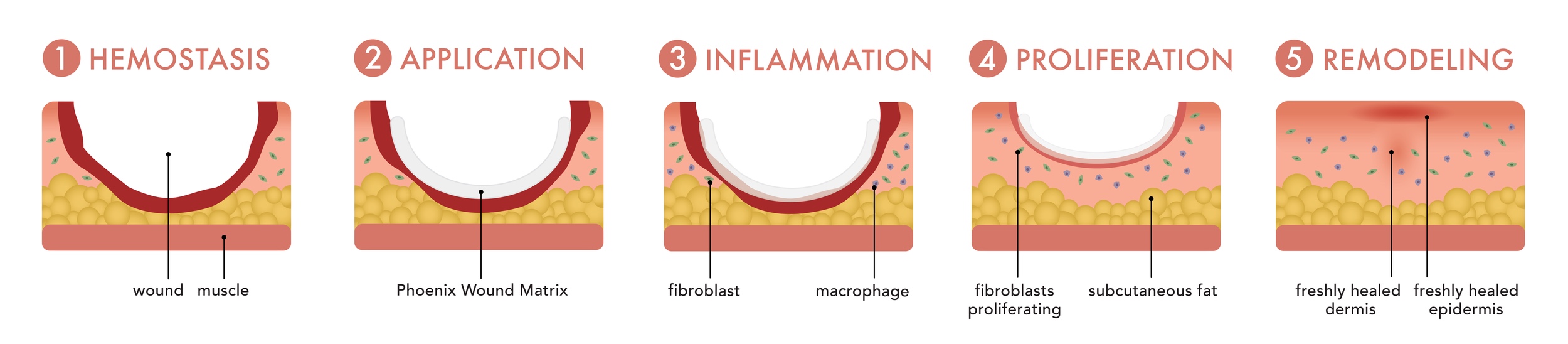
Electrospun Scaffolding Wound Healing Case Study
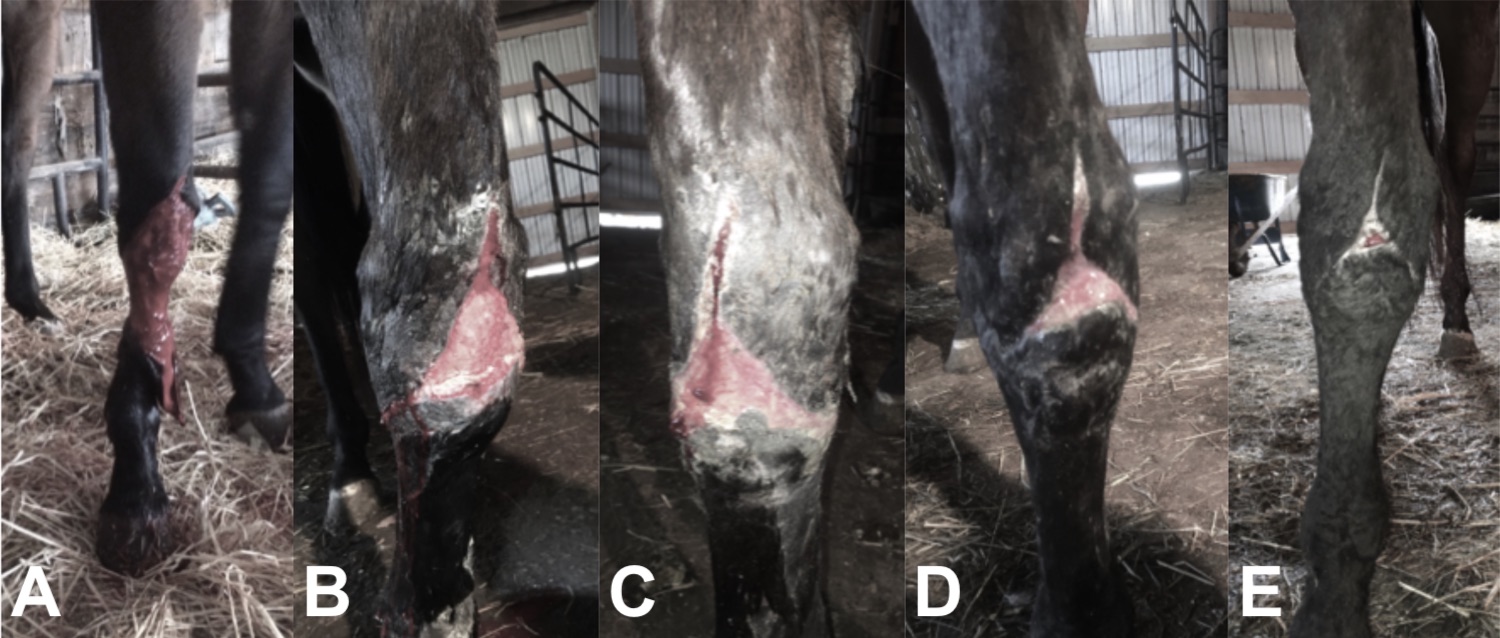
The picture below shows an animal that was treated with Phoenix Wound Matrix, an electrospun graft made with our LE-500. When the veterinarian saw the severity of the wound in this animal (image ‘a’), the recommendation was humane euthanasia. The team at Renovoderm knew that wound healing was possible with the use of a biocompatible and biodegradable material made through electrospun fibers. Treatment with the FDA approved Phoenix Wound Matrix proved that regeneration of natural tissue was possible (images ‘b’ through ‘e’). This electrospun material is able to biomimic the ECM environment, accelerate the healing process, reduce scar formation, allow normal tissue growth and regeneration, and reduce painful dressing changes while being fully resorbable.
Electrospinning technique, the future at your hands
Home-built units are commonly used in research labs to create electrospun material for tissue engineering and regenerative medicine applications. However, when advance research, product development, or pre-industrial commercialization is needed, home-built units do not meet the requirements. The Fluidnatek technology allows the tight control of fiber dimensions and stability of fiber/particle production. Controlling the environmental parameters during the spinning process is required to achieve consistent, homogeneous and reproducible results – especially in tissue engineering applications. The units can be installed with an environmental control unit; while the solvents are removed from the cabinet, temperature and humidity are consistently maintained. The temperature of this environmental control unit can be maintained from 18 to 45°C, and the relative humidity from 10 to 80 %.
All units are scalable, which allows the technique to be scaled to a production plant. As a matter of fact, Bioinicia has the first and only GMP certified plant for the production of fibers and particles for pharmaceutical applications employing the Fluidnatek technology. The LE-100 and LE-500 have the capabilities for current good manufacturing process (cGMP) certification for your tissue engineering application needs and has demonstrated material creation that can be approved by the FDA. The use of the Fluidnatek technology is a breakthrough, not only in regenerative medicine and tissue engineering but also other applications used around the world.
