One of the primary contributors to climate change is the increased concentration of carbon dioxide (CO₂) in the atmosphere, mainly from burning fossil fuels, deforestation, and industrial processes. CO₂ is a greenhouse gas that traps heat in the Earth’s atmosphere, leading to global warming, rising sea levels, and extreme weather events. Reducing CO₂ emissions and developing technologies to capture and utilize CO₂ are critical to mitigating climate change and its impacts on human health.
CO₂ electrolysis can help combat climate change by reducing atmospheric carbon dioxide levels and repurposing CO₂ as a valuable resource. It enables a circular carbon economy by converting CO₂ into useful chemicals and fuels like carbon monoxide, methane, or ethanol. This process reduces reliance on fossil fuels for these products, thereby lowering overall emissions. When powered by renewable energy, CO₂ electrolysis becomes a carbon-neutral technology, simultaneously mitigating climate impacts while providing sustainable energy and chemical solutions.

Carbon Dioxide Electrolysis:
CO₂ electrolysis is a process that uses electrical energy to convert carbon dioxide (CO₂) into valuable chemicals and fuels, such as carbon monoxide (CO), methane (CH₄), ethylene (C₂H₄), or ethanol (C₂H₆O). This process typically occurs in an electrochemical cell, where CO₂ is reduced at the cathode while water or another chemical is oxidized at the anode (Figure 1). CO₂ molecules are broken down via electrochemical reduction, driven by an external voltage applied to the system.1 Specialized catalysts (e.g., metals like copper, silver, or nickel) are used to modify the free energy landscape to favor the reduction reaction and to tune the product selectivity.
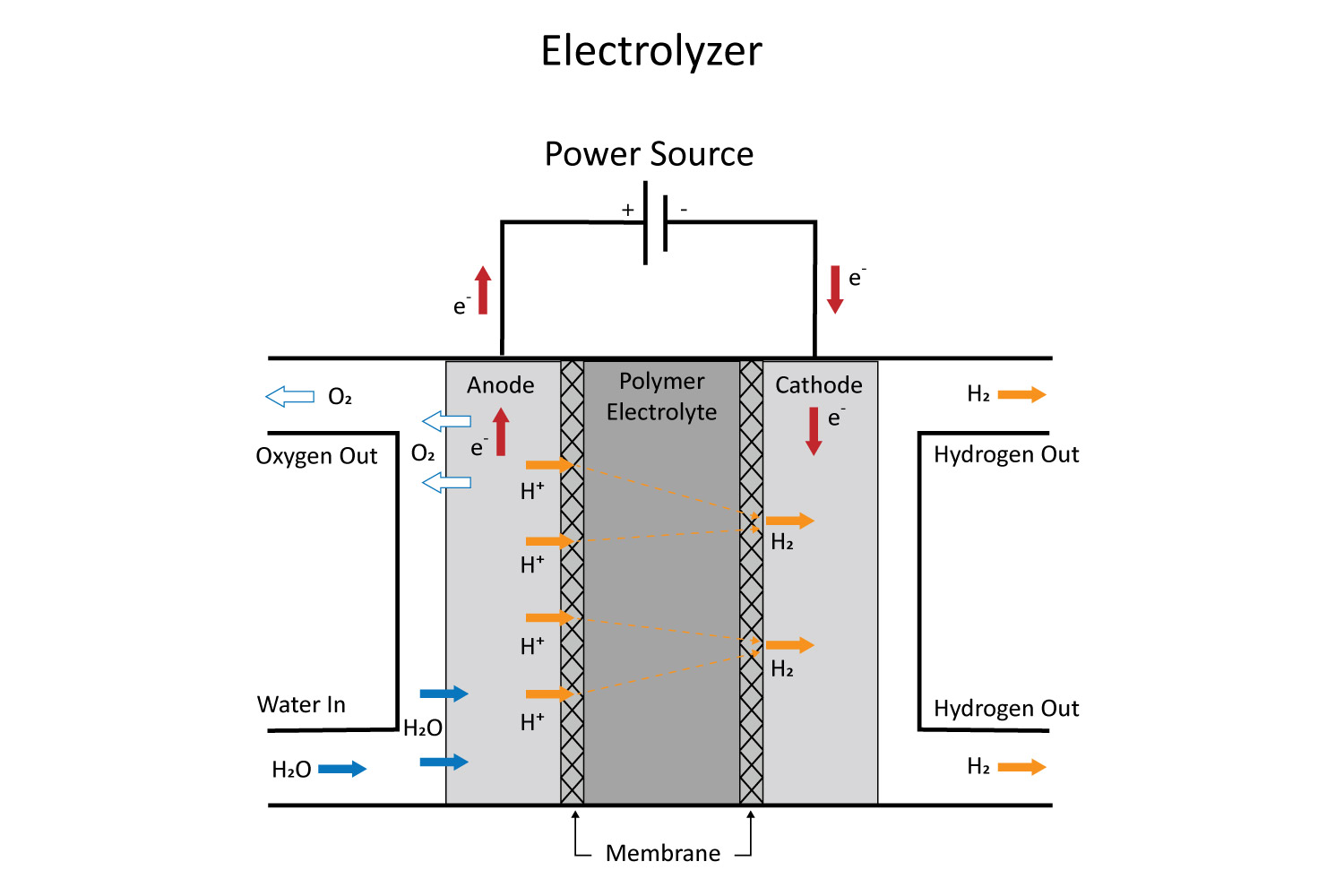
Despite its potential, CO₂ electrolysis faces several challenges. Achieving high efficiency electrolysis, where the fraction of energy used to produce the desired product is minimized, remains challenging. Developing catalysts that are selective, durable, and cost-effective continues to be a key area of research. Transitioning from laboratory-scale systems to industrial-scale processes requires addressing engineering and economic barriers.
Nanoparticles as Efficient Catalysts:
One approach to develop highly efficient catalysts is to use nanoporous surfaces composed of small primary nanoparticles. Nanoparticles are highly efficient catalysts due to their large surface-area-to-volume ratio, which provides more active sites for chemical reactions compared to bulk materials. Their nanoscale dimensions allow for unique electronic, optical, and catalytic properties that enhance reaction rates and selectivity. Additionally, nanoparticles can be engineered with precise shapes, sizes, and compositions to tailor their catalytic performance for specific reactions.
Nanoporous layers fabricated using small nanoparticles will have an exceptionally high surface area, enhancing their performance. The pore size and distribution can be precisely controlled by adjusting the size, shape, and assembly of the nanoparticles, enabling customization for specific applications. Nanoporous layers also offer increased reactivity and selectivity for chemical processes, particularly in catalysis and adsorption, due to their high density of active sites. These attributes make them ideal for applications in energy conversion and chemical synthesis where high efficiency and cost-effectiveness are essential.
Spark Ablation
Spark ablation is a physical process used to produce nanoparticles by vaporizing material from a solid electrode using high-voltage electrical sparks. A high-voltage electrical discharge is applied between two conductive electrodes made of the material to be ablated. The intense heat from the spark vaporizes small amounts of the electrode material (Figure 2). The vaporized material cools and condenses into nanoparticles, which are carried by a gas flow (often inert gases like argon) to be collected or directly used. This technique is widely used for producing nanoparticles in research and industrial applications due to its simplicity and scalability.2
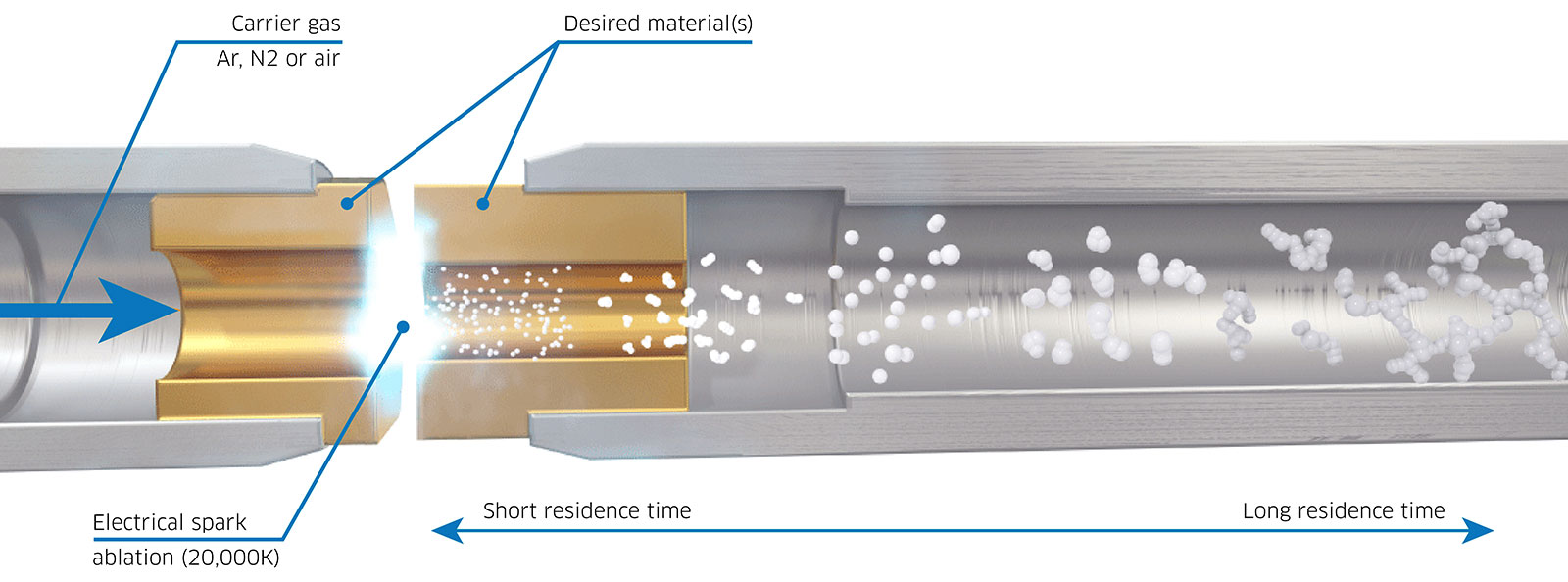
Since no chemical precursors or solvents are used, the produced nanoparticles are free from contamination and capping agents, ensuring high purity and catalytic activity. Spark ablation can produce nanoparticles from a broad range of materials, including metals, alloys, ceramics, and even composites. The particle size and distribution can be tuned by adjusting operational parameters like gas type, flow rate, or spark energy. The process is solvent-free and often uses inert gases, making it more environmentally friendly compared to chemical synthesis methods. Spark ablation systems are relatively simple to design and can be scaled for industrial production of nanoparticles.
VSParticle Technology
VSParticle technology offers a novel approach to CO2 electrolysis catalyst development by enabling the rapid and precise synthesis of alloy catalysts using spark ablation technology.
- Material and Substrate Agnostic: VSParticle technology is adaptable to a wide range of elements and substrates, eliminating the need for tailored synthesis methods required by traditional wet chemistry techniques. This allows for a more streamlined and versatile approach to material discovery.
- Production of Advanced Nanoporous Layers: The technology excels in producing the smallest stable nanoparticles, crucial for creating advanced nanoporous layers. These layers offer a high surface area, increasing the number of active sites for catalytic reactions.
- Combinatorial Printing Capability: The system allows for the rapid fabrication and testing of diverse material combinations, accelerating the discovery of optimal catalysts. This combinatorial approach enables efficient screening of large datasets, essential for identifying promising catalyst candidates.
- Reproducible and consistent: The automated and precise layer-by-layer printing process ensures reproducibility and consistency. This has the potential to address a key challenge in transitioning lab-scale discoveries to real-world applications.
- Elemental Composition Control and Automated Printing: The VSParticle nanoprinting system allows precise control over the elemental composition and structure of printed catalyst layers. Researchers can fine-tune these parameters to optimize catalyst performance.
- Low-Temperature Annealing Process: After printing, a low-temperature annealing process can be applied to enhance material properties by altering the internal atomic arrangement due to the low melting point of the small nanoparticles. This process promotes alloy formation while preserving the nanoporous structure, maximizing the number of active catalytic sites.
Conclusion
CO₂ electrolysis is a transformative technology with the potential to combat climate change, enhance renewable energy utilization, and revolutionize chemical production. VSParticle technology is not only accelerating the discovery of novel and efficient CO2 electrolysis catalysts but is also paving the way for their scalable production. The collaboration between VSParticle and Meta for the Open Catalyst Experiments 2024 (OCx24) project highlights the technology’s impact.3 VSParticle’s ability to rapidly synthesize diverse materials contributed to the creation of a vast experimental database. Continued research and development will be critical to overcoming the challenges and unlocking the technologies full potential in creating a sustainable, low-carbon future.
References
- Ozden, A., García de Arquer, F.P., Huang, J.E. et al. Carbon-efficient carbon dioxide electrolysers. Nat Sustain 5, 563–573 (2022). https://doi.org/10.1038/s41893-022-00879-8 ↩︎
- Koolen, C.D., Pedersen, J.K., Zijlstra, B. et al. Scalable synthesis of Cu-cluster catalysts via spark ablation for the electrochemical conversion of CO2 to acetaldehyde. Nat. Synth 4, 336–346 (2025). https://doi.org/10.1038/s44160-024-00705-3 ↩︎
- Abed, J., Kim, J., Shuaibi, M., et al. Open Catalyst Experiments 2024 (OCx24): Bridging Experiments and Computational Models. arXiv preprint arXiv:2411.11783 (2024). https://doi.org/10.48550/arXiv.2411.11783 ↩︎








