Cyclic voltammetry (CV) is one of the most popular techniques used for electrochemistry experiments and is commonly used to study oxidative and reductive processes. This technique involves measuring the current that is generated during the redox process when the voltage is varied between the electrodes. CV can be used to assess the redox properties, charge-discharge mechanisms, and electrochemical stability of materials. Cyclic voltammetry can distinguish reversible, quasi-reversible, and irreversible behavior in electrochemical systems.
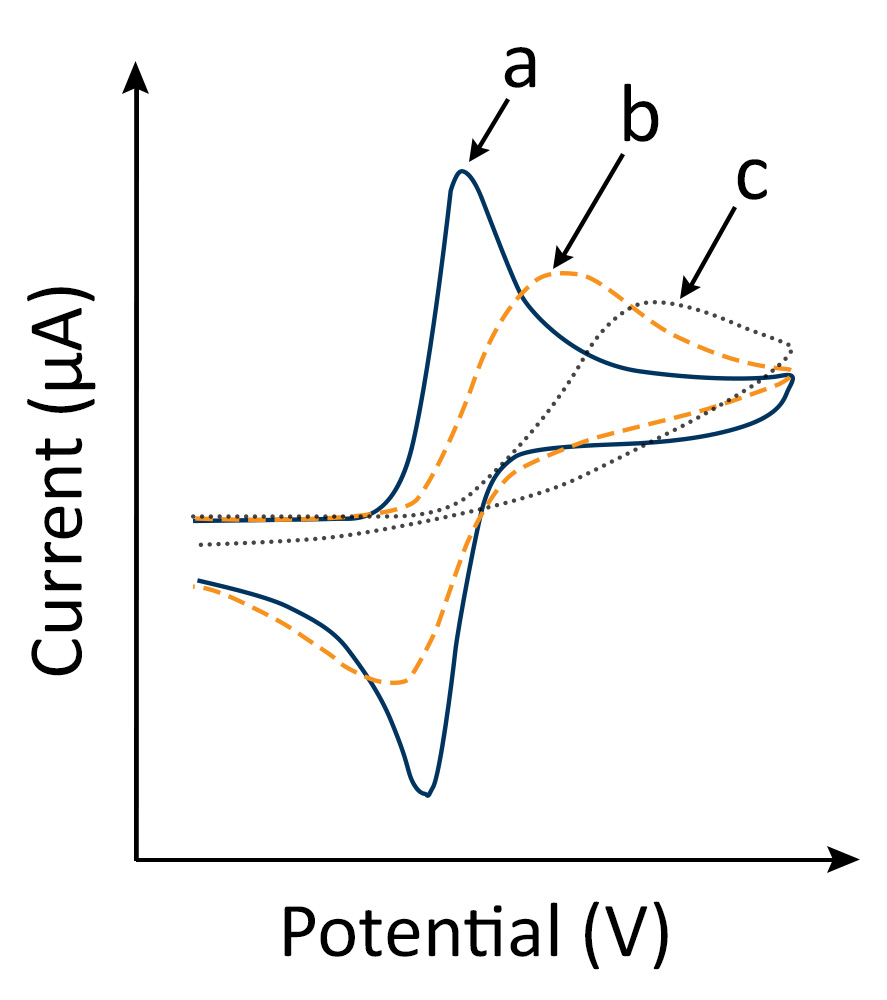
A CV experiment involving solution reactants results in a “duck-shaped” plot known as a cyclic voltammogram (Fig. 1). The scan rate dependence of peak heights in cyclic voltammograms allows for the evaluation of the dominant control mechanism for electron transfer.
Since the thermodynamic state of a battery is controlled via the potential, CV is routinely used to study batteries and battery materials. In addition, since the current flow indicates the reaction rate, kinetic information may be extracted. CV can help determine the safe voltage range within which a material can operate without experiencing undesirable side reactions and enhance the stability and cycle life of the battery. Hence, Cyclic Voltammetry can be employed for rapid screening and evaluation of candidate materials for battery components.
In this blog, we summarize the use of cyclic voltammetry in the study of battery materials.
1. Electrolytes
The electrolyte controls the degree of parasitic reactions that result in the growth of the Solid Electrolyte Interface (SEI). This growth governs the capacity retention and lifetime aspects of the battery’s performance. Cyclic voltammetry has been used to examine the behavior of electrolytes in lithium and sodium ion batteries and to identify electrolyte decomposition via irreversible behavior [1]. Variation of the potential limits of the cyclic voltammogram allows for the identification and association of reduction and new oxidation peaks with each other giving additional insight into the decomposition process. Information about the nature of the decomposition products can be obtained. This study demonstrated that the scan rate dependence of the decomposition peaks can be used to verify the surface attachment of the decomposition leading to SEI formation. The CV technique presented in the study could potentially be used as a screening and optimization method for electrolyte additives.
2. Cathode Materials
Cathode material evaluation can use cyclic voltammetry to probe the reactions of the material [2]. One study investigated the electrochemical behavior of LiFePO4 at different temperatures. The scan rates for these investigations are very low (compared to dissolved electroactive species) due to the thick layers of material that are deposited, but still show the classical behavior. Thick layers are used to mimic the use in an actual battery. Scan rates in the microvolt per second range are typically used. This equates roughly to similar charge/discharge rates that are observed for constant current experiments. For example, to scan over a 600 mV range at an equivalent charge rate of 0.1C requires a scan rate of 16.6 microvolts/s. The scan rate dependence of the redox peaks of the cathode materials elucidates the dominant factors controlling the charge transfer process. Temperature-dependent studies allow for the evaluation of the activation energy associated with the charge transfer process through the compiling of an Arrhenius plot. This study demonstrated that both the electron transfer activity and the lithium-ion diffusion rate in the LiFePO4 electrode increased with increasing operating temperature.
3. Anode Materials
Cyclic voltammetry has been extensively used in the study of anode materials particularly concerning replacements for graphitic materials and research into other alkali metal battery systems.
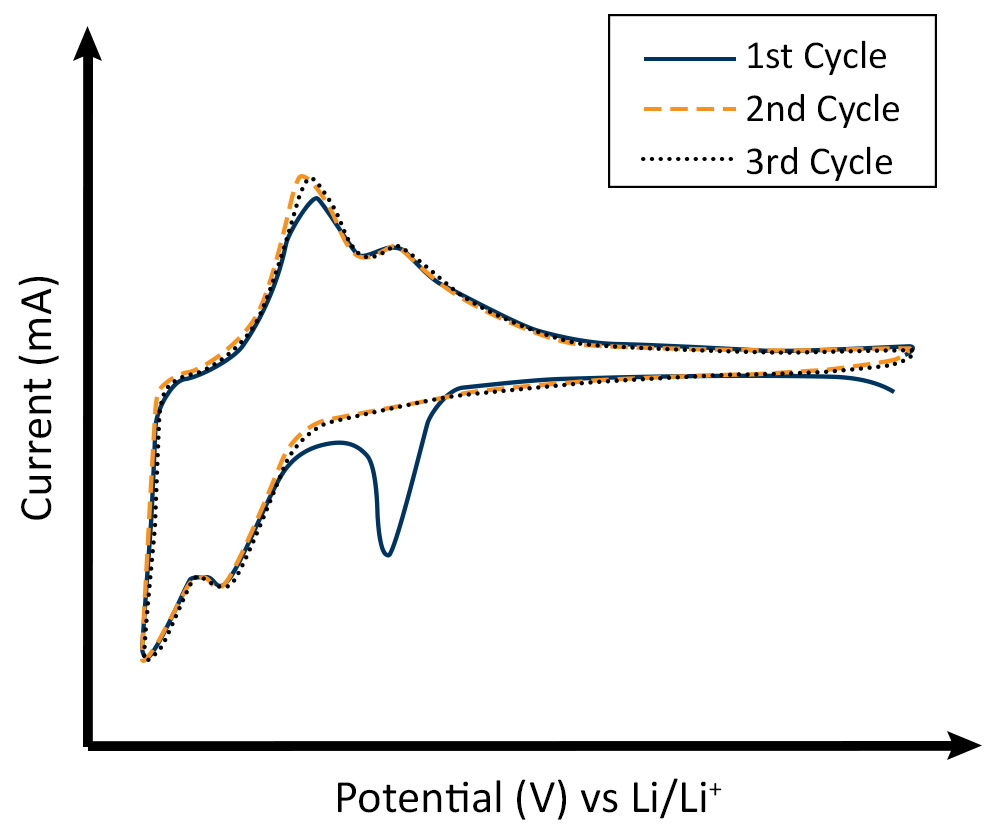
CV was used to investigate the SEI formation on silicon as an anode material for high-energy lithium-ion batteries [3]. Initial voltammograms of the behavior often show the irreversible peak associated with the Solid Electrolyte Interface (SEI) formation which can also be connected to a SEI formation plateau in the galvanostatic cycling of the anode as they occur at similar voltages (Fig 2). The amount of charge measured for this formation peak yields an estimate for the material deposited. Scan rate studies are also applicable in this area and yield information about the main charge transfer process. The separation of the faradaic and capacitive sections of the cyclic voltammogram provides information about the overall charge storage process.
Conclusion
Cyclic voltammetry is a fundamental tool used in the development and understanding of the components that make up rechargeable batteries like Lithium-ion batteries. Analysis of peak heights, shapes, and positions helps identify the important processes. Rate information can also be obtained especially regarding ion diffusion.
References
[1] Claudio Cometto et al., Means of Using Cyclic Voltammetry to Rapidly Design a Stable DMC-Based Electrolyte for Na-Ion Batteries. Journal of The Electrochemical Society, 166 (15) (2019) A3723-A3730
[2] Masaya Takahashi et al., Reaction behavior of LiFePO4 as a cathode material for rechargeable lithium batteries. Solid State Ionics 148, (2002) 283 – 289
[3] Chen, L.B., Xie, J.Y., Yu, H.C. et al. An amorphous Si thin film anode with high capacity and long cycling life for lithium-ion batteries. J Appl Electrochem 39, (2009) 1157–1162